Need for Antioxidants in Adhesives
Need for Antioxidants in Adhesives
Antioxidants are used in a variety of adhesive formulations to protect against degradation caused by reaction with atmospheric oxygen. The increasingly demanding requirements which modern adhesives are expected to meet, can only be achieved and retained by using appropriate stabilizer systems.
Adhesives and their raw materials react like other organic materials with oxygen in a process called "autoxidation". Autoxidation is initiated by various factors, such as:
- Heat
- Light
- Mechanical stress
- Catalyst residues, or
- Reaction with impurities
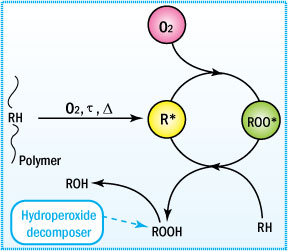
Free radicals are formed and react in the presence of oxygen to form peroxy radicals, which further react with organic material leading to hydroperoxides (ROOH).
These reactions ultimately lead to a change in chemical composition and polymer properties, such as molecular weight, resulting in immediate impact on adhesive's mechanical, aesthetic, or bonding properties that determine the service life of an adhesive. They key properties which can be impacted include:
- Discoloration
- Viscosity changes
- Char formation
- Cracking and
- Loss of adhesion
When used in adhesives formulations, antioxidants work by interrupting the degradation process in different ways. They help to preserve quality and performance of adhesive formulations during all its life cycle.
Oxidation of Adhesives - When & Why Does it Occur?
Oxidation of Adhesives - When & Why Does it Occur?
Oxidation can occur at all stages of an adhesives life from synthesis to final end-use. It is usually recognized at high processing temperatures such as during mixing, compounding, or extrusion (in the case of hot-melt adhesives). However, oxidation can also occur at relatively low temperatures including ambient storage and also on exposure to UV light.
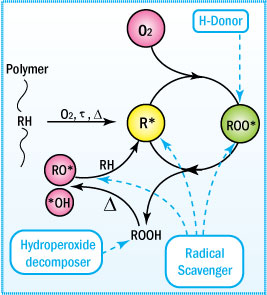 Oxidation of Adhesives
Oxidation of Adhesives
| Life Cycle Stage |
Common Reason for Degradation |
| Raw material manufacturing |
- High temperature treatment
- Catalyst residues
- Shipping processes
|
| Raw materials storage and shipment
|
- Prolonged exposure to oxygen at ambient
temperatures
|
| Adhesive manufacturing |
- High temperature processing
- Shear
- Oxygen exposure
|
| Application of adhesive to substrate
|
- High temperature processing
- Oxygen exposure
|
| Service life |
- Prolonged exposure to oxygen at ambient
or elevated temperatures
- Exposure to UV light
|
Characteristics of Oxidation During an Adhesive's Typical Life Cycle
Components Susceptible to Oxidation
Polymers that are commonly used in adhesive formulations differ considerably in their sensitivity to oxidation. For example:
- Acrylates are highly resistant to oxidation and do not generally require antioxidants at ambient temperatures
- Unsaturated elastomers are highly susceptible to oxidative decomposition and require relatively high concentration of antioxidants for protection.
Adhesive components especially susceptible to oxidation include base synthetic polymers like ethylene vinyl acetate & styrene block copolymers, polyolefins, polyamides, natural rubber, polychloroprene, polyurethane, and butyl rubber.
Hydrocarbon additives, such as tackifiers and waxes, are also vulnerable to oxidation and can actually contribute to the oxidation of the base polymer. Metallic and other impurities in the adhesive can accelerate the oxidation process. Depending on the aging environment, most adhesives can benefit from antioxidants.
Role of an Antioxidant
The function of an antioxidant is to prevent the propagation of oxidation. Antioxidants interrupt the oxidation process in different ways according to their chemical structure. The antioxidant is usually optimized for a specific polymer or combination of polymers and for a specific environment or market segment.
As a result, there are many different families of commercial antioxidants that are specifically designed to meet the particular needs of the adhesives industry.
The role of antioxidants in providing longer life has taken on added importance with the growth of hot-melt adhesives and structural adhesives that must perform in elevated temperature environments.
Mechanism of Uninhibited Autoxidation
Mechanism of Uninhibited Autoxidation
To understand the function of an antioxidant, one must
first understand the
mechanism of degradation.
- Oxidative degradation starts
with the initiation of free radicals on exposure to heat, UV radiation,
and mechanical shear or in the presence of reactive impurities such as
catalyst residues.
- These radicals attract neighboring labile hydrogen
atoms to produce unstable hydroperoxides plus additional free radicals
that keep the process going.
This mechanism is generally characterized
by three distinct stages: initiation, propagation, and termination. These
steps are shown and described below.
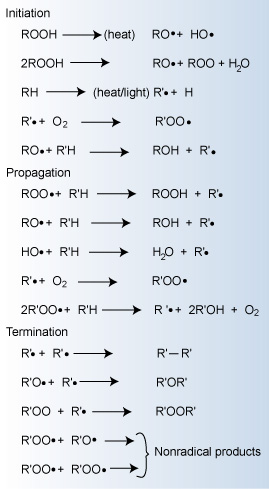 Major reactions occurring during polymer oxidation
Major reactions occurring during polymer oxidation
This cycle leads to degradation of the polymer. Only when a non-radical
inert product is formed will the cycle be terminated. In preventing this
type of degradation, antioxidants function by intercepting the radicals
or by preventing radical initiation during the polymer's life cycle.
Initiation
Free radical initiation can be produced by several processes related
to the input of some form of energy. Polymeric free radicals (R")
can be formed by exposure to heat, light, and shear. Impurities and catalyst
residue can also lead to the generation of free radicals, which then rapidly
react with oxygen to form peroxy radicals.
It is virtually impossible to manufacture commercial polymers that do
not contain traces of hydroperoxides. The peroxide bond is relatively
weak and cleaves easily to yield alkoxy or hydroxy radicals. Once oxidation
has started, the concentration of hydroperoxides becomes appreciable,
and their decomposition is the main source of radical initiators.
The absorption of UV light also produces radicals by cleavage of hydroperoxides
and carbonyl compounds. Most polymer degradation caused by the absorption
of UV light results from radical initiated autoxidation. Thus,
UV degradation
and degradation from oxidation are tightly linked and often dealt with
together.
Propagation
The propagation reactions involve the very rapid reaction of the free
radicals with oxygen forming peroxy radicals (RCOO"). The peroxy
radicals can then react with the polymer to produce hydroperoxides (ROOH),
which can further decompose to react with the polymer to produce free
radical species. Thus, the mechanism (often termed autoxidation) is cyclic
and can be repeated many times before being terminated. It leads to an
exponential growth of free radicals.
The rate of oxidation is usually very low at the start of aging and increases
with time. Trace quantities of transition metal ions greatly accelerate
the rate of oxidation by catalyzing the decomposition of the hydroperoxides
into radical species. The most operative metal ions are copper, iron,
cobalt, and manganese.
Termination
Conversion of radicals to non-radical species terminates the propagation
reactions. The termination mechanisms generally result in crosslinking
or chain scission. Thus, the resulting ramifications of oxidation include
those physical effects summarized in the Table below. A change in color (yellowing)
is also often evident from oxidation.
| Property change to crosslinking |
Property change due to chain scission |
| Hardening |
Softening |
| Skinning |
Decrease in viscosity |
| Gel formation |
Increase in tack |
| Decrease in tack |
Loss of cohesive strength |
| Increase in viscosity |
Property Change Due to Crosslinking and Chain
Scission Resulting from Oxidation
Fortunately, there are a number of stabilization chemistries that disrupt and inhibit the autoxidation cycle by preferentially and sacrificially reacting with the radical species and hydroperoxides. Protection of the adhesive components from degradation is achieved by obstructing one or more of the oxidation mechanisms described above.
The specific antioxidants and concentrations employed have an important
impact on the physical properties of the adhesive.
Antioxidants are generally
added to each raw material component of an adhesive formulation that is
prone to oxidation, and in most cases, they are also added to the final
adhesive formulation. The use of antioxidants only in the final formulation
process will not undo any oxidative damage that might be caused to the
raw materials via storage, processing, etc.
Let's understand the different classes of
antioxidants in detail.
Classification of Antioxidants
Classification of Antioxidants
Antioxidants interrupt the degradation process in different ways, according to their structure. The major classifications of antioxidants are listed below:
- Primary Antioxidants – They are chain-breaking antioxidants which react rapidly with peroxy radicals (ROO*).
- Secondary Antioxidants – They react with hydroperoxides (ROOH) to yield non-radical, non-reactive products and are also called as hydroperoxide decomposers.
- Multi-functional Antioxidants – They combine both primary and secondary antioxidant functions in one compound, have only recently become available
- Hydroxylamines – They are capable of scavenging C-radicals and act as both primary and secondary antioxidants.
- Carbon-centered Radical Scavengers – They are effective in trapping alkyl radicals and provides powerful processing stability.
Primary Antioxidants
Primary antioxidants function by donating their reactive hydrogen to the peroxy free radicals so that the propagation of subsequent free radicals does not occur.
Mechanism of Antioxidation
- Primary or free radical scavenging antioxidants inhibit oxidation via chain terminating reactions.
- They have reactive OH or NH groups.
- Inhibition occurs via a transfer of a proton to the free radical species.
- The resulting radical is stable and does not abstract a proton from the polymer chain.
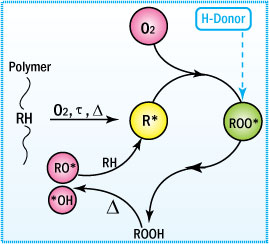 Mechanism of Antioxidation of Primary Antioxidants
Mechanism of Antioxidation of Primary Antioxidants
The two major groups among the primary antioxidants are hindered phenolics and aromatic amines.
Hindered Phenols
Phenolic stabilizers are primary antioxidants that act as hydrogen donors. They react with peroxy radicals to form hydroperoxides and prevent the abstraction of hydrogen from the polymer backbone. Often used in combination with secondary antioxidants, phenolic stabilizers are offered in an extensive range of molecular weights, product forms, and functionalities.
Sterically hindered phenols, are the most widely used stabilizers of this type. They are effective during both processing and long term thermal aging and many have FDA approvals.
These products generally resist staining or discoloration. However, they may form quinoid structures upon oxidation, which leads to yellowing. Phenolic antioxidants include simple phenolics, bisphenolics, polyphenolics, and thiobisphenolics.
Hindered phenolics, such as butylated hydroxy toluene (BHT), high molecular weight phenolics, and thiobisphenolics, are the most popular of the primary antioxidants. They are suitable as long-term stabilizers in almost all cases.
-
BHT has long been the workhorse of the antioxidant industry, and it possesses FDA approval for food contact. However, BHT is a relatively volatile material that is gradually being replaced by higher molecular weight antioxidants that resist migration.
-
Thiobisphenolics can also act as peroxide decomposers (secondary antioxidants) at temperatures above 100°C. Therefore, they are often found in higher temperature applications.
ROO* radicals are deactivated by hindered phenol via the following reaction.
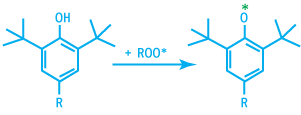 Deactivation of RCOO* Radicals
Deactivation of RCOO* Radicals
The phenoxy radical generated are very stable due to their ability to build numerous mesomeric forms.
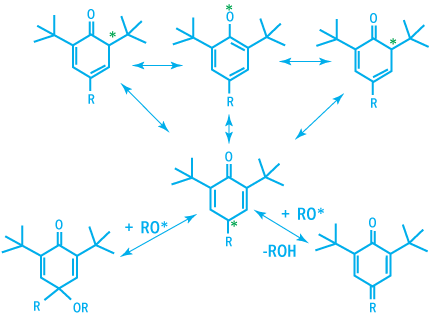 Mesomeric Forms of Phenoxy Radicals
Mesomeric Forms of Phenoxy Radicals
Secondary Aromatic Amines
Secondary aromatic amines act as primary antioxidants and are excellent hydrogen
donors.
 Mechanism of Secondary Aromatic Amines
Mechanism of Secondary Aromatic Amines
Also available in an extensive range of molecular weights and product forms, aromatic
amines are often more active than hindered phenols, because of less steric hinderance.
Aromatic amines, however, are more staining & discoloring than hindered phenols, especially
on exposure to light or combustion gases (gas fade) and have limited FDA approvals
for use in contact with food.
Amines are commonly used in the rubber industry, but they also find uses in applications such as wire and cable formulations and in polyurethane polyols.
Secondary Antioxidants
Secondary antioxidants are used in conjunction with primary antioxidants to
provide added stability to the polymer. They destroy the unstable hydroperoxides
that function as sources of free radicals during oxidative degradation.
They react with hydroperoxides to yield non-reactive products. Thus, secondary
antioxidants are also known as hydroperoxide decomposers.
Hydroperoxide decomposers prevent the split of hydroperoxides into extremely reactive alkoxy and hydroxy radicals. Organophosphorus compounds and Thiosynergist antioxidants are widely used hydroperoxide decomposers.
Mechanism of Antioxidation
- Secondary antioxidants decompose hydroperoxides into non-radical, non-reactive, and thermally stable products.
- They are often used in combination with primary antioxidants to yield synergistic stabilization effects.
 Mechanism of Antioxidation of Secondary Antioxidants
Mechanism of Antioxidation of Secondary Antioxidants
Organophosphorus Compounds
Organophosphorus compounds are secondary antioxidants that decompose peroxides and hydroperoxides into stable, non-radical products. They are extremely effective stabilizers during processing and are normally used in combination with a primary antioxidant.
Trivalent phosphorus compounds are excellent hydroperoxide decomposers. Generally, phosphites (or phosphonites) are used and react according to the following general reaction, generating phosphates.
 Phosphites (or Phosphonites) React to Generating Phosphates
Phosphites (or Phosphonites) React to Generating Phosphates
Some of these compounds are sensitive to water and can hydrolyze, leading to formation
of acidic species. While the addition of an acid scavenger can minimize the effect,
the industry has generally converted to hydrolysis-resistant compounds.
Commercially available phosphites differ by the nature of the aryl groups. Some
typical structures are shown below.
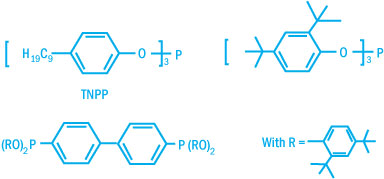 Commercially Available Phosphites
Commercially Available Phosphites
Thiosynergists
Among sulfur-based hydroperoxide decomposers, esters of 3,3-thiodipropionic acid
play an important role. Thiosynergists react according to the following general
reaction, generating sulfoxides and sulfones.
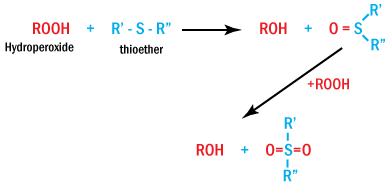 Thiosynergists Reaction Generating Sulfoxides and Sulfones
Thiosynergists Reaction Generating Sulfoxides and Sulfones
Although thiosynergists do not improve the melt stability of polymers during polymer
processing, they are very efficient for long-term thermal aging applications. Sulfur-based hydroperoxide decomposers are mainly used in combination with
hindered phenol antioxidants.
The most common commercially available thiosynergists are based on either lauric
or stearic acid.
The table below lists the chemical types of common primary and secondary
antioxidants and their major resin applications.
|
Suitable Polymer Applications |
Comments |
| Primary Antioxidants |
|
Amine |
Rubber, some pigmented polymers, and polyurethane polyols |
Arylamines tend to discolor and cause staining |
| Phenolic |
Polyolefins, styrenics, and most engineering resins |
Phenolics are generally stain resistant and include simple phenolics (BHT), various polyphenolics, and bisphenolics |
| Secondary Antioxidants |
| Organo-phosphite |
Polyolefins, styrenics, and most engineering resins |
Phosphites can improve color stability and property retention, but can be corrosive if hydrolyzed |
| Thioester |
Polyolefins and styrenics |
The major disadvantage with thioesters is their odor which is transferred to the host polymer |
Common Antioxidants by Chemical Type with Major
Resin Applications
Multifunctional Antioxidants
Due to their special molecular design, multifunctional antioxidants optimally combine primary and secondary antioxidant
functions in one compound.
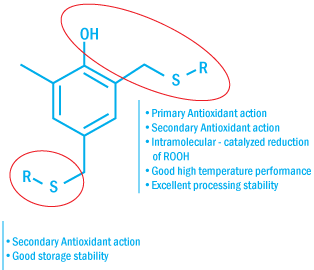 Structure of Multifunctional Antioxidants
Structure of Multifunctional Antioxidants
Having several stabilizing functions combined in the same molecule, multi-functional
antioxidants eliminate the need for co-stabilizers, such as phosphites and thioethers.
This not only simplifies the formulation, but it also simplifies the storage, handling,
and use of the stabilizer.
Hydroxylamines
Hydroxylamines may act as both primary and secondary antioxidants, providing processing
stability, comparable to phenol/phosphite systems. In addition, they provide excellent
light stability when used in combination with hindered amines and are resistant
to gas-fade discoloration. They are capable of scavenging C-radicals.
The mechanism of radical scavenging by hydroxylamine is shown below.
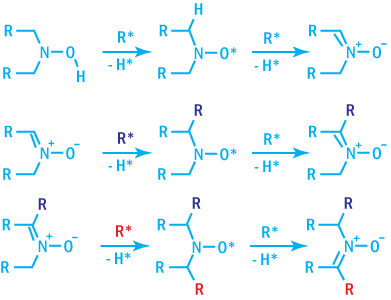 Mechanism of Radical Scavenging by Hydroxylamines
Mechanism of Radical Scavenging by Hydroxylamines
Carbon-centered Radical Scavengers
Carbon-centered radical scavengers are effective in trapping alkyl radicals, provides powerful processing stability.
Mechanism of Antioxidation
Radical scavengers are antioxidants capable of trapping radicals. Scavenging of
alkyl radicals would immediately inhibit the autoxidation cycle. Under oxygen deficient
conditions alkyl radical scavengers contribute significantly to the stabilization
of the polymer. Scavenging the extremely reactive alkoxy and hydroxy radicals is
practically not possible.
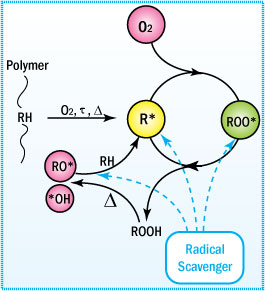 Antioxidation Mechanism of Radical Scavengers
Antioxidation Mechanism of Radical Scavengers
Carbon centered radical scavengers, such as lactones and acrylated bis-phenols, are extremely effective in oxygen deficient environments.
Lactones
Lactones (Benzofuranone derivatives) are powerful radical scavengers. Even when
added in small amounts, they help control melt stability during polymer processing.
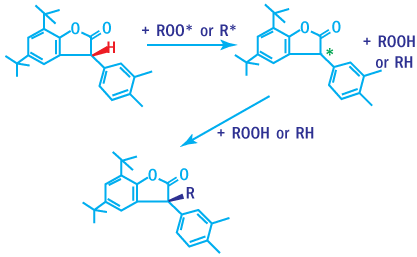 Antioxidation Mechanism of Lactones
Antioxidation Mechanism of Lactones
Substituted benzofuranone are excellent radical scavengers. They are mainly used
in combination with phenolic compounds and phosphite to provide materials excellent
performance even at low concentrations.
Acrylated Bis-Phenols
Acting as traditional hindered phenols, acrylate functionalized hindered phenols
are efficient C-radical scavengers.
Antioxidation Mechanism of Acrylated Bis-Phenols
They are very effective in preventing styrene copolymers from crosslinking or degrading
during processing, particularly under oxygen deficient conditions. They are usually
used in conjunction with other stabilization chemistries.
Benefits of Antioxidants
Benefits of Antioxidants
As adhesives or adhesive components can undergo degradation during its life cycle, the main benefits of antioxidants like color stabilization, viscosity stabilization, or retention of adhesive properties will the preserve quality and performance of adhesive formulations.
Any factor in the life cycle leads to an undesirable change in physical properties of the unstabilized adhesives, creating manufacturing problems, poor product appearance and reduction of adhesive strength. The role of antioxidants is to prevent or retard all these undesirable changes.
Color Stability
A common undesirable effect of degradation is discoloration. Both natural polymer materials and synthetic polymers in adhesives &sealants undergo induced discoloration as an effect of degradation. Typically observed by an increase in yellowness or darkening, discoloration often appears prior to any measurable loss in physical properties.
It is usually the first critical indicator of damage that is observed, which determines the useful service life of the product.
Although color development occurs at widely differing degrees in various adhesives
& sealants and may not directly correspond to a decrease in the mechanical properties,
a change in appearance is often unacceptable in many adhesive applications.

|

|

|
|
Adhesive without AO
|
Adhesive with AO-1
|
Adhesive with AO-2
|
Positive Effect of Antioxidant Systems on Color Stability of an Adhesive
after Heat-aging at 170°C
Although not illustrated, additional benefits of antioxidants may include improved
initial color of the adhesive prior to thermal aging.
Viscosity Stability
An important requirement for adhesives and their raw materials is viscosity stability. It is essential through all stages of the product's life cycle:
- Processing
- Storage
- Shipment
- Use
- Aging
In formulated adhesives, the resins, plasticizers, and other polymers present must be protected.
Depending on the predominant degradation mechanism, the product may either decrease or increase in viscosity if not stabilized. For polymers where cross-linking predominates, an increase in viscosity is often accompanied by gel formation or skin formation. Chain scission, leading to a molecular weight decrease on aging manifests itself by a decrease of viscosity.
The most appropriate stabilizer combination will depend on the selection of other formulation ingredients, as well as on the aging conditions. Suitable phenolic antioxidants in combination with thiosynergists or multifunctional antioxidants exhibit the best performance.
Skin Formation
For certain hot-melt adhesives, high temperature aging may lead to skin formation. This occurs with the cross-linking reaction. Skinning is based on irreversible oxidative damage to the polymer and cannot be corrected by subsequent mixing or addition of stabilizers.
During processing, skin formation in the adhesive can lead to blocking of pipes and irregular coating. The addition of the proper additive package during the manufacturing of the hot melt significantly retards skin formation.
 High Temperature Aging in HMAs May Lead to Skin Formation
High Temperature Aging in HMAs May Lead to Skin Formation
Note: Originally atmospheric conditions become anaerobic under a cross linked skin, due to reduced diffusion of oxygen.
Retention of Adhesive Properties
From a product use standpoint, the most important characteristic affected by degradation
is adhesive performance. It is not only necessary to maintain the adhesive's ability
to adhere, but also to ensure that the adhesive bond exhibit minimal change in adhesion/cohesion
properties over the service life of the adhesive. These essential properties can
be adversely affected if the adhesive system is not adequately protected from oxidative
degradation.
The changes in bulk mechanical properties often observed in adhesives and their
components reflect either oxidative cross-linking and/or chain scission. For adhesives
and sealants, the consequences of degradation via
cross-linking include:
- Loss of tack and peel strength
- Embrittlement
- Decrease of solubility
- Loss of elongation
- Hardening of the polymer
Some polymers that predominantly degrade via cross-linking are – polyethylene, EVA, polymers that contain butadiene (i.e. SBR, SBS), and tackifier resins..
Degradation via chain scission
often results in:
- Increase in tack
- Loss of cohesive strength
- Embrittlement
- Loss of physical properties
- Softening of the polymer
Polymers that predominantly degrade via chain scission are polypropylene, polymers that contain isoprene (i.e. NR, SIS), and butyl rubber.
Through the proper choice of antioxidant or antioxidant combinations, the loss of
adhesion or adhesive properties due to degradation can be avoided or at least minimized.
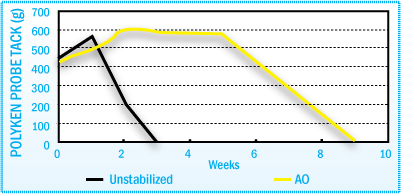 Stabilization of HMA Polyken Probe Tack After Film Aging at 70°C
Stabilization of HMA Polyken Probe Tack After Film Aging at 70°C
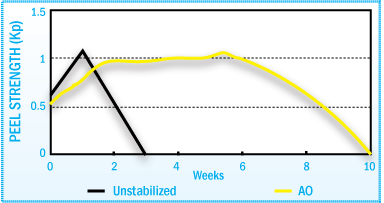 Stabilization of HMA Peel Strength After Film Aging at 70°C
Stabilization of HMA Peel Strength After Film Aging at 70°C
The figures above illustrate how the use of antioxidants in a SBS hot-melt adhesive
can help maintain adhesive performance throughout the aging cycle.
Having learnt about different types of antioxidants available and
their benefits, now explore the use of antioxidants in different types of
adhesive and sealant formulations.
Antioxidants for Hot-melt Adhesives
Antioxidants for Hot-melt Adhesives
Hot-melt adhesives and the components used in their manufacture are generally solid
or highly viscous at room temperature. As a result, hot-melt adhesives are exposed
to elevated temperatures, which are required to melt the components, during both
formulation and end use. When exposed to elevated temperatures the hot-melt adhesives
become susceptible to thermo-oxidative degradation.
| Signs of adhesives degradation during formulation and processing |
Signs of degradation during end use |
- Skinning
- Viscosity changes
- Discoloration
- Char Formation
|
- Loss of adhesion
- Loss of cohesive strength
- Loss of peel strength, etc.
- Discoloration
|
Antioxidants play a key role in the hot-melt systems utilizing polymers are discussed below.
Recent developments in the bulk delivery and storage of hot-melt adhesives have
allowed manufacturers and their customers to increase productivity, reduce costs
and decrease waste. To make this possible, the adhesive is stored and kept at elevated
temperatures of greater than 100°C.
Chemical market offers a wide range of stabilizers
and stabilizer systems to ensure that the quality and performance of the adhesive
is protected during shipment, through storage and use.
Ethylene copolymers (EVA, EBA, EMA, etc.)
Hot-melts based on ethylene copolymers (EVA, EBA, EMA, etc.) are generally used
in the packaging industry as quick-sealing adhesives, in disposables, for book and
journal binding, and in the wood working industry as assembling adhesives.
There is a strong trend towards high heat resistant and low color hot-melt adhesives.
Low color is perceived as a good indicator of adhesive quality. It is influenced
by the choice of raw materials, as well as by the stabilizer package.
Effectively stabilized raw materials are critical for the production of hot-melts
with constant and reproducible properties. However, this does not mean that the
basic stabilization is sufficient to overcome degradation occurring during compounding
and end use.
Additional Stabilization Package for Better Performance
An additional stabilization package is required to retard discoloration, viscosity
changes, and skinning of EVA hot-melts during compounding at high temperatures.
Irganox® 1010, a hindered phenol antioxidant, provides sound performance with improvements attainable with the use of phosphite co-additives.
For example, stabilized EVA hot-melts based on hydrocarbon resin show good color stability
after oven aging at 170°C. (See figure below)
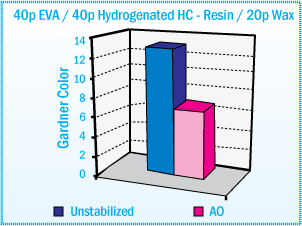 Gardner Color of EVA Hot-Melt Adhesives Based
on Hydrocarbon Resin After Aging at 170°C
Gardner Color of EVA Hot-Melt Adhesives Based
on Hydrocarbon Resin After Aging at 170°C
During high temperature aging of hot-melts, skin formation and increased melt viscosity can occur. Skinning is
based on irreversible oxidative damage to the polymers and cannot be corrected by
subsequent mixing or addition of stabilizers. During processing, skin formation
in the adhesive can lead to blocking of pipes and irregular coating.
As shown in
the figure below, the addition of additives significantly retards skin formation, with no
skin formation after 72 hours at 170°C, and provides excellent viscosity stability.
A significant decrease in melt viscosity of ethylene copolymer based hot-melt adhesives can also occur on aging. This problem can also be significantly reduced with the addition of antioxidants.
In EVA hot-melt adhesives, the addition of 25% hindered phenol (Irganox® 1010) and 0.25% phosphite (Irgafos® 168) was found to significantly reduce the decrease in melt viscosity during aging at 170°C.1
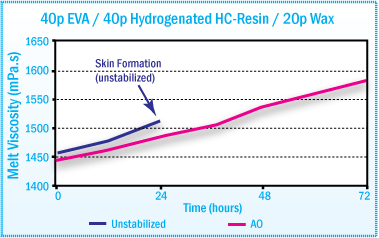 Melt Viscosity of EVA based Hot-Melt After
Aging at 170°C
Melt Viscosity of EVA based Hot-Melt After
Aging at 170°C
Various tackifiers such as hydrocarbons, rosin esters, terpene resins, or dicyclopentadienes, or other additives used in the EVA hot-melt adhesive formulation generally do not affect the choice of antioxidant.
Styrene Block Copolymers
SIS-, SBS-, and SEBS- / SEPS- based adhesives have enjoyed strong growth in the
pressure sensitive adhesive market, including tape and packaging applications, as
well as other markets.
Adhesive formulations based on unsaturated elastomers such as SIS and SBS are more
sensitive to oxygen, ozone, light and heat than those based on EVA. Although the
use of a nitrogen blanket offers some protection during mixing, additional stabilization
is essential to protect the adhesives against oxidative degradation during preparation,
storage and end use.
The following sections will focus on the potential degradation and necessary stabilization
of SIS, SBS, SEBS hot-melt adhesives during some of the main steps of
their product life cycle:
- Hot-melt manufacture, simulated by dynamic aging
- Hot-melt coating, simulated by static aging at high temperatures
- Hot-melt end use, simulated by static aging at low temperatures
Stabilization of SIS Hot-Melt Adhesives
A hindered phenol antioxidant such as Irganox® 1010 is considered to be the workhorse of the antioxidant in the adhesives industry and especially in SIS hot-melt adhesives for all-around performance. The additions of a phosphite co-antioxidant will generally provide further improvement in color stability.
Dynamic Aging
During compounding, SIS hot-melt adhesives are subjected to high heat and high mechanical
shearing, which induce chain scission of the SIS polymer. This results in a lower
melt viscosity of the hot melt. To limit the melt viscosity decrease and the discoloration
of the hot melt, it is necessary to use a high concentration of stabilizers, over
1%, possibly up to 3%.
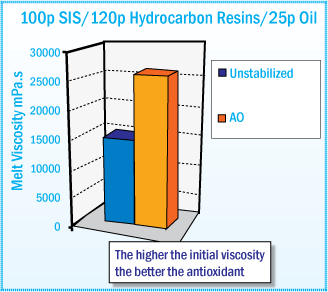 Initial Melt Viscosity After Mixing at 170°C
Initial Melt Viscosity After Mixing at 170°C
Static Aging at High Temperatures
During static aging at high temperatures, you can see excellent color retention
(See figure below).
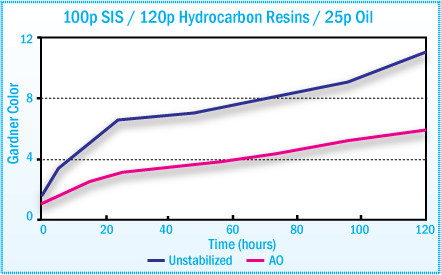 Discoloration of SIS Hot-Melt Adhesives During
Oven Aging at 170°C
Discoloration of SIS Hot-Melt Adhesives During
Oven Aging at 170°C
Stabilization of SBS Hot-Melt Adhesives
SBS polymers typically experience increased viscosities as a consequence of oxidation. The phenol-phosphite blend antioxidants provide consistent, stable viscosity over the duration of high processing temperatures, whereas systems with no antioxidant and those with phenol-only undergo significant viscosity change.
A new antioxidant, Irganox® 1726, has been found to provide significant improvements in HMA type applications. This antioxidant combines both phenol and thio-type moieties in the molecule. The interaction of these groups within the same molecule yields a very potent antioxidant.
Stabilization of SEBS Hot-Melt Adhesives
Although SEBS is inherently more stable than either SBS or SIS, it too requires an appropriate stabilization system. A stabilization package consisting of 0.5% hindered phenol (Irganox® 1010) and 0.5% phosphite (Irgafos® 168) will provide
improvements in color, reduced changes in viscosity, and postponing skin formation.
Polyolefins
APAO polymers are used as a base for the manufacture of a wide variety of hot-melt
adhesive and sealant systems. Because APAO is compatible with many solvents, tackifiers,
waxes, and other polymers, it is well suited for many adhesive applications in the
packaging, construction, medical, and personal care industry.
As an example, APAO
hot-melt adhesives are widely used for hygienic applications in the adhesive industry
such as disposable diapers.
Similar to styrene-isoprene systems, APAO polymers degrade via chain
scission during high temperature aging. The appropriate choice of
antioxidant or antioxidant system can prevent the effects of degradation
such as color formation and most importantly, viscosity decrease.
Phenolic antioxidants provide very good viscosity control, but use of co-stabilizers such as phosphites can provide some additional improvements with regard to color, while still maintaining good viscosity control.
Dynamic Aging at High Temperatures
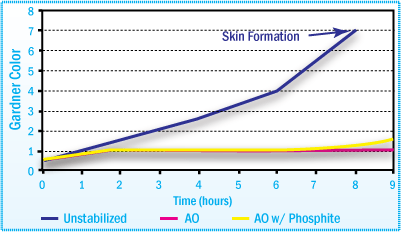 Gardner Color of APAO-HMA after Aging at 200°C
in Mixer
Gardner Color of APAO-HMA after Aging at 200°C
in Mixer
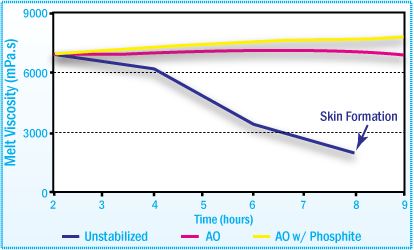 Viscosity of APAO-HMA after Aging at 200°C
in Mixer
Viscosity of APAO-HMA after Aging at 200°C
in Mixer
Static Aging at High Temperatures
The use of a phosphite in conjunction with the primary antioxidant
improves the long-term color stability of the APAO hot-melt adhesive.
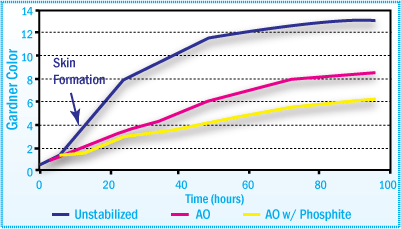 APAO-HMA Color After Aging at 170°C
APAO-HMA Color After Aging at 170°C
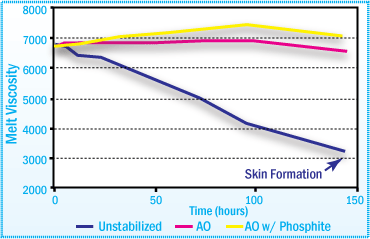 APAO-HMA Viscosity After Aging at 170°C
APAO-HMA Viscosity After Aging at 170°C
Polyamides
Polyamide hot-melt adhesives are used in a variety of industrial applications. Possessing a broad range of adhesive properties, most polyamide adhesives are characterized
for exhibiting:
- Excellent adhesion to a wide variety of substrates
- Bonding well
to porous substrates
- Good chemical and oil resistance, and
- Good adhesion over a wide range of service temperatures.
Most polyamides, particularly the dimer acid polyamides, are prone to oxidation
and must be stabilized by antioxidants. Although the stabilizers will vary with
the polyamide, the most common antioxidants that are used are hindered phenols,
phosphites, phosphates, and hindered aromatic amines. Generally a blend of antioxidants
is used.
Phenolic antioxidant, is able to stabilize polyamide hot-melt adhesives against
degradation occurring at high temperatures. These antioxidants will contribute to improved initial color and long term thermal stability, particularly at lower end-use temperatures.
Alternative stabilizers are aromatic
amine, which may be used either alone or in combination with other antioxidants. Check out the advantages and disadvantages of these antioxidant systems in the table below.
|
Antioxidant System
|
Advantages
|
Disadvantages
|
|
Phenolics
|
- Best performance at lower temperatures
- Good initial and long-term color performance
|
- Less effective at high temperature conditions such as under-the-hood auto applications
|
|
Aromatic amines
|
- Good long-term thermal performance
|
- Discoloration
- Requires relatively high concentrations
|
Antioxidant Systems for Polyamide Hot-Melt Adhesives
Antioxidants for Solvent-based Adhesives
Antioxidants for Solvent-based Adhesives
Solvent-based adhesives principally consist of polymers such as natural rubber, polychloroprene, etc., dissolved in aliphatic or aromatic solvents.
Natural and chloroprene rubbers are unsaturated elastomers and are therefore susceptible to attack by oxygen and light. Although they are not exposed to very high temperatures during processing, as is the case in the preparation of hot-melt adhesives, they do need to be stabilized against degradation in end use, especially when used in thin films.
Therefore, stabilizers should be added in the formulation to protect the adhesive during both processing (drying) and service life. There are products in market which are effective antioxidants for such applications:
- Natural rubber-based adhesives
-
Polychloroprene-based adhesives
Natural Rubber-based Adhesives
Loss of adhesive properties and color formation are important parameters to be measured
after film aging.
Films obtained from the following formulation, 100 parts NR, 80 parts rosin ester
and 1000 parts toluene, are aged at 70°C for several days. In figures below
you can see, better tack retention and improved color retention.
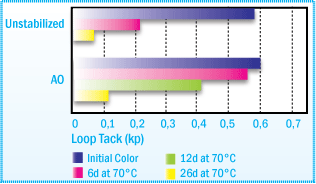 Loop Tack (PSTC-5) of NR solvent-based Adhesive
After Film Aging at 70°C
Loop Tack (PSTC-5) of NR solvent-based Adhesive
After Film Aging at 70°C
Adhesive films (-35 Nm) coated on polyester foils are aged covered with silicon
paper. All values are averages of 3 replicates (± 10% relative).
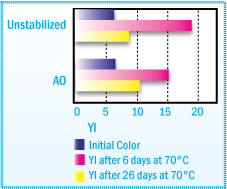 Yellowness Index of NR Solvent-based Adhesive
After Film Aging at 70°C
Yellowness Index of NR Solvent-based Adhesive
After Film Aging at 70°C
Polychloroprene-based Adhesives
Polychloroprene is one of the most versatile materials used as a base for elastomeric adhesives. Contact adhesives made of chloroprene films develop high bond strength, high tack level, and auto-adhesion.
To determine the appropriate antioxidant and antioxidant level to use, it is necessary
to conduct testing based on the application requirements. Non-staining, non-discoloring
antioxidants are recommended. They offer overall good stabilizer performance.
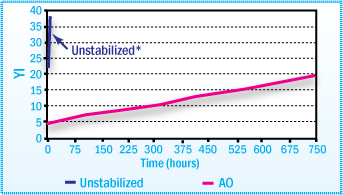 Yellowness Index of CR SBA Films After Aging
at 80°C
Yellowness Index of CR SBA Films After Aging
at 80°C
» Discover Antioxidants for Solvent-based Systems Here!
Masking Tape Adhesive Formulation
The table below shows a typical formulation
for a masking tape employing an antioxidant additive.
|
Component
|
Part per hundred of resin (phr)
|
|
Natural rubber
|
100
|
|
Polyterpene
|
41
|
|
Calcium carbonate
|
58
|
|
Resole phenolic resin
|
51
|
|
Antioxidant (butylated hydroxytoluene,
BHT)
|
2
|
|
Solvent (hexane:toluene, 70:30)
|
450
|
Masking Tape Adhesive Formulation
Antioxidants for Water-based Adhesives
Antioxidants for Water-based Adhesives
Water-based adhesives consist mainly of polyvinylacetate (PVA), polyacrylate, natural rubber, carboxylated-SBR, chloroprene rubber, polyurethane, etc.
Most water-based adhesives need to be stabilized to achieve and maintain good properties. Liquid additives allow easy preparation of emulsions and are therefore particularly well-suited for these applications.
Depending on the type of water-based adhesive applications, solid additives may outperform after heat aging:
-
Natural rubber water-based adhesives
-
Carboxylated-SBR water-based adhesives
Natural Rubber Water-based Adhesives
The time to penetration into paper is an important criterion for envelope manufacturers,
and this time can be significantly increased (See figure below).
 Time to adhesive Failure After Film Aging
at 105°C of NR Water-based Adhesive
Time to adhesive Failure After Film Aging
at 105°C of NR Water-based Adhesive
Coated on Paper, Aged Uncovered.
Carboxylated-SBR Water-based Adhesives
For adhesives based on carboxylated-SBR (X-SBR) latex, a universal stabilization
system is a mixture 2 antioxidants in the ratio 1:1. This combination shows good
synergism, preventing discoloration and retention of tack properties during aging. (See figures below)
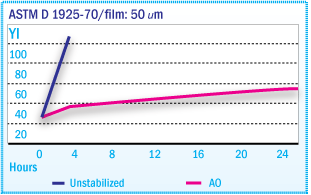 Yellowness Index of X-SBR Films During Oven
Aging at 135°C
Yellowness Index of X-SBR Films During Oven
Aging at 135°C
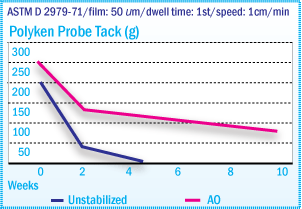 Polyken Probe Tack of X-SBR Films After Oven
Aging at 70°C
Polyken Probe Tack of X-SBR Films After Oven
Aging at 70°C
» Explore the Antioxidants for Water-based Adhesives Here!
Antioxidants for Reactive Adhesives
Antioxidants for Reactive Adhesives
Reactive adhesives are typically characterized by the formation of permanent bonds
between substrates to provide resistance to chemicals, moisture and heat. These
adhesives exhibit high bond strength and long-term durability under severe environmental
conditions.
Categories of reactive adhesives include:
- Hot-melt
Polyurethanes
- Solvent based
Polyurethanes
- Epoxies
- Modified Acrylics
- Anaerobics
- Cyanoacrylates
- Radiation Curable
Hot-Melt Polyurethanes
Reactive hot-melts, typically combine the traditional good properties of conventional
hot-melts with the well-known characteristics of polyurethane adhesives. This combination
results in products with enhanced performance.
Unlike conventional thermoplastic
adhesives, which remelt when heated, PUR hot-melts cure to a thermoset material,
retaining their structural integrity once cured.
In addition, the cured adhesive
has excellent temperature and environmental resistance, generally withstanding exposure
to temperatures from -30°C to +150°C, while maintaining strong bonds between
similar and dissimilar substrates.
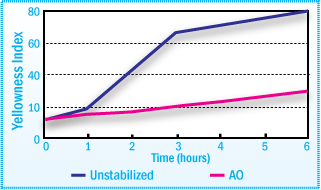 Yellowness Index (Aging at 130°C)
Yellowness Index (Aging at 130°C)
As with other hot-melts, PUR hot-melt adhesives are also exposed to thermal oxidative
influences and in part, also to mechanical stress caused by shear. The rapid discoloration
of an unstabilized PUR hot-melt adhesive is evident in the figure above.
Solvent-based Polyurethanes
Polyurethane adhesives are produced in many grades such as one-component, two-component, dispersion and solvent-based, for use in different application areas. Solvent-based polyurethane adhesives are suitable for a number of different industrial and construction applications, having good adhesion to rubber, leather, textiles, metal, paper, wood, and plastics. In addition, solvent-based polyurethane adhesives are used for a wide variety of laminating applications.
As with other adhesives, stabilizers should be added in the formulation to protect the adhesive from degradation during both processing and service life. Some antioxidants are effective for solvent-based polyurethanes.
Antioxidants for Sealants
Raw Material Stabilization
Raw Material Stabilization
Market demand for improved adhesive systems has resulted in a concurrent demand for the improved quality of the main components of many of these systems.
Stabilization of key comnponents of adhseives promote retention of adhesion properties, viscosity and color are critical during long term storage and adhesive manufacturing. Let's discuss different polymer stabilization, tackifier stabilization and role of waxes.
Stabilizing Polymers (EVA, SIS, SBS, SEBS)
EVA Stabilization
After exposure to temperatures up to 170-200°C, typical adhesive grade ethylene vinyl acetate (EVA) polymers (vinyl acetate content: 18-40%) undergo discoloration together with gel formation and a viscosity increase. During static aging at 170°C, the viscosity increase can be reduced by the use of relatively low levels of antioxidants. The same antioxidants also reduce gel formation after dynamic aging (Figure 1) and hinder color development.
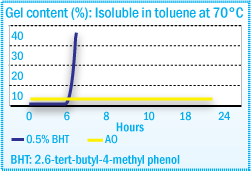 Gel Content of EVA After Compounding in a Sigma Blade Kneader at 170°C
Gel Content of EVA After Compounding in a Sigma Blade Kneader at 170°C
The stabilization of neat EVA resin generally with by 2,6,-di-t-butyl-p-cresol (BHT) antioxidant provides an unsatisfactory level of performance for the hot-melt adhesive formulator. The presence of skinning and discoloration requires additional antioxidant to meet the processing requirements of hot-melt adhesives. Hindered phenols, such as Irganox® 1010, will provide the stability required, eliminating skin and gel formation and discoloration.
SIS Stabilization
Unsaturated elastomers such as styrene-isoprene-styrene (SIS) and styrene-butadiene-styrene (SBS) block copolymers contain double bonds in their structure. Therefore, they are more sensitive to oxidation than saturated polymers. SIS and SBS, however, behave quite differently during degradation as illustrated in the figure below. SBS is predominantly cross-linked by oxidation, leading to gel formation (torque increase), whereas SIS displays mainly chain scission, leading to molecular weight decrease (torque decrease).
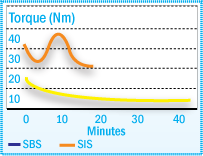 Torque Development of SIS & SBS at 180°C in a Brabender Plasticorder
Torque Development of SIS & SBS at 180°C in a Brabender Plasticorder
The difference in the degradation mechanisms must be considered when preparing hot-melt adhesives. Generally a combination of hindered phenols and phosphites provide the optimum thermal stability in these applications.
Controlling the degradation of neat SIS during mixing at high temperatures and under
high shear forces is more difficult than SBS. As a result, it is necessary to use
an effective processing stabilizer, stabilizer system, or a multifunctional antioxidant
to efficiently stabilize neat SIS under these conditions.
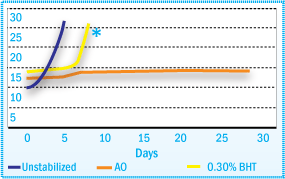 Melt Flow Index of SIS After Oven Aging at
70°C
Melt Flow Index of SIS After Oven Aging at
70°C
*Sample containing BHT is completely destroyed after seven days aging: MFI cannot
be measured (sample is almost liquid).
SBS/ SEBS/ SEPS Stabilization
SBS copolymers, as with EVA, have the tendency to crosslink under thermo-oxidative
conditions. Simulating compounding conditions in a Brabender Plasticorder, cross-linking
is observed as an increase in torque (viscosity). Induction time, which is the time
to an increase in torque of 1Nm after the minimum, is a useful parameter for evaluating
the processability of SBS.
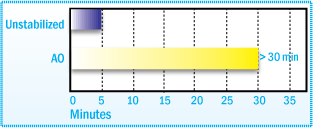 Induction Time After Brabender Plasticorder Aging of SBS at
160°C
Induction Time After Brabender Plasticorder Aging of SBS at
160°C
As with SIS polymers, a higher molecular weight, more effective stabilizers, such as 2,4-bis(n-octylthio)-6-(4-hydroxy-3,5-di-tertbutylanilino)- 1,3,5-triazine (Irganox® 565), used alone or in combination with a phosphite antioxidant provides superior color stability and resistance to gel formation.2
SEBS and SEPS based hot-melt adhesives are inherently more stable than SBS or SIS. Although antioxidants may not be necessary for the raw material, hot-melt formulations generally require an appropriate stabilization system for protection during melt processing.
Stabilizing Tackifiers
Natural Tackifiers Stabilization
Rosin-based tackifiers find continued use in hot-melt adhesives because of their
unique property of providing compatibility to a wide variety of adhesive components.
Unmodified rosins, however, are easily oxidized on account of their main constituent,
abietic acid (See figure below), containing a conjugated double bond (a), tertiary carbon
atoms (b), and allylic hydrogen atoms (c).
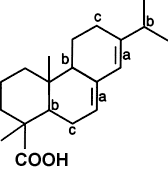 Sites of Potential Oxidation (Abietic Acid)
Sites of Potential Oxidation (Abietic Acid)
Because of their tendency to degrade, natural rosins must be modified to meet the
stability requirements of the hot adhesives. Improved stability can be developed
by several processes such as hydrogenation, disproportionation, and dimerization,
followed by esterification of the rosin with glycerol or pentaerythritol.
Esterification Catalyst and Bleaching Agent
Various catalyst systems such as TNPP, calcium acetate, zinc acetate, and zinc oxide
are used to accelerate the esterification reaction times. Catalyst offers the shortest reaction time and produces a resin with reduced color
and lower acid number. In addition, they impart a "carry through" antioxidant
effect by offering improved heat stability of the final resin.
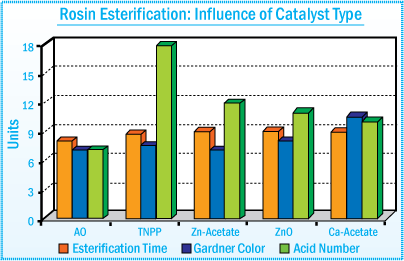
Rosin Esterification : Influence of Catalyst
Type
As shown in the autoxidation mechanisms of adhesives, hydroperoxides are formed
during degradation. These hydroperoxides are stable at ambient temperatures, and
may accumulate in the raw material during storage. Upon heating, they may decompose
and induce further degradation. Therefore, the determination of hydroperoxide content
is a good method for quality control for these materials.
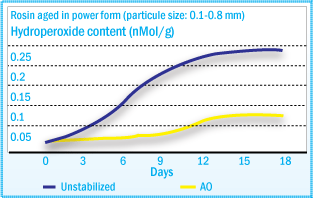 Hydroperoxide Formation of Rosin Ester During
Aging
Hydroperoxide Formation of Rosin Ester During
Aging
Hydroperoxide content correlates well with melt viscosity and color changes after
heat treatment – ROOH formation (See figure above) and color development (See
figure below) present
similar profiles during oven aging. The color increase of tackifiers after heat
treatment is caused by hydroperoxide decomposition and subsequent chromophore formation.
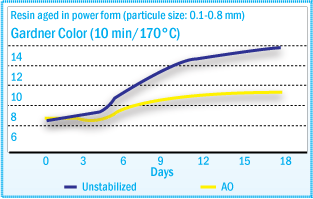 Gardner Color of Rosin Ester During Aging
at 40°C
Gardner Color of Rosin Ester During Aging
at 40°C
A suitable stabilizer system should therefore be used to provide long-term oxidation
resistance to the tackifier and to ensure consistency of its key properties. In static oven agent at 40°C of a rosin ester tackifier, the rate of hydroperoxide formation can be reduced significantly using a hindered phenol (Irganox® 1010), with even better results using Irganox® 5652.
Synthetic Tackifiers Stabilization
Synthetic hydrocarbon resins make up another major group of tackifier resins. Synthetic
tackifier resins consist of hydrocarbon resins (C5-, C9-, aliphatic or aromatic),
a collection of modified or special resins, primarily phenolics, and polyterpenes.
As with rosin esters, synthetic tackifiers tend to oxidize during shipment and storage
unless they contain an antioxidant. This oxidation is usually observed as an accumulation
of hydroperoxides, which greatly influences tackifier properties that relate to
end-use performance. The addition of a hindered phenol antioxidant such as Irganox® 1010 can greatly reduce the hydroperoxide formation during static oven aging at 40°C.
The figure below exhibits hydroperoxide formation in a C5-aliphatic hydrocarbon resin after
low temperature aging at 40°C.
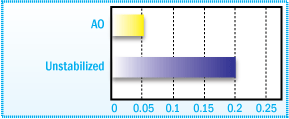 Hydroperoxide Formation in a C5-aliphatic
Hydrocarbon Resin after 15 Days Oven Aging at 40°C
Hydroperoxide Formation in a C5-aliphatic
Hydrocarbon Resin after 15 Days Oven Aging at 40°C
Because hydroperoxides lead to discoloration at high temperatures, the use of antioxidants
can also prevent discoloration during long-term storage.
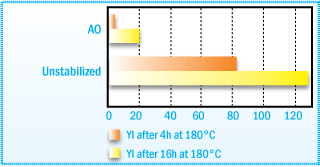 Yellowness of a Hydrogenated Hydrocarbon Resin
After Oven Aging at 180°C.
Yellowness of a Hydrogenated Hydrocarbon Resin
After Oven Aging at 180°C.
Other benefits that are obtained with the use of antioxidants are excellent viscosity
stability and skin prevention. These results are applicable for other types of synthetic
tackifier resins as well. It is strongly recommended that all tackifier resins (natural or synthetic) should
be protected by the addition of stabilizers as early as possible in their life cycle,
i.e. in the resin manufacturing process, to prevent hydroperoxide formation during
storage.
Waxes
The two main reasons to include waxes in hot-melt adhesive formulations are to lower cost and reduce viscosity.
Generally included at levels of 20-30% in hot-melt adhesive formulations, properties
affected by the wax content are blocking characteristics, softening point, and open
time. The high melting microcrystalline waxes (m.p. 90°C) and synthetic waxes
(m.p. 100-120°C) are used because they contribute to high temperature properties
and greater cohesive strength. The high melting paraffin waxes (m.p. 65-70°C)
are used extensively in hot-melt coatings for their barrier, anti-blocking and heat
seal properties, as well as their lower cost.
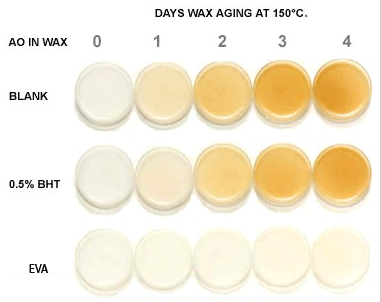
Influence of wax preaging on EVA hot-melt initial color
As with other organic materials, waxes are susceptible to autoxidation and
will lose their original properties if overlooked. Please refer the attached photo
illustrating, there is not only a significant improvement in the initial color of
the wax, but also in the overall color retention of the wax after aging. Other benefits
include enhanced viscosity stability and adhesive properties, all contributing to
the improved performance of the final hot-melt adhesive.
Various antioxidants can be used to improve the thermal stability of waxes. However, the use of a high molecular weight, less volatile antioxidant such as Irganox® 1010 significantly outperforms the more volatile antioxidants during high temperature processing.
Selection of Antioxidants
Selection of Antioxidants
The selection of an antioxidant system is mainly dependent upon the polymer to be stabilized. For example, polypropylene is less stable than the polyethylene and will require a more effective stabilization package. In addition, the anticipated melt processing temperature and the expected service temperature will determine the type of antioxidant that should be used.
Below mentioned are crucial parameters that need to be considered when selecting an antioxidant for any particular adhesive formulation:
Other parameters that must be considered during antioxidant selection include:
Let's visit each factor separately.
Compatibility and Solubility
Nearly all polymeric materials require the addition of antioxidants to retain physical properties and to ensure adequate service life. The nature of the polymeric resin and the solubility of the antioxidant in the polymer will influence the kind and amount of antioxidants used. The table below links common antioxidant families that are generally used with base polymers in the adhesive formulation.
| Base Polymer |
Antioxidant Type |
| Amines |
Hydro-quinones |
Phenolics |
Phosphites |
Thioesters |
| Acrylics and acrylic copolymers |
|
|
X |
X
|
|
| Epoxies (EP) |
X |
|
|
|
|
| Ethylene co- and ter-polymers: Solids (EVA, EMA) |
X |
|
X |
X |
X |
| Natural rubber (NR) |
X |
|
X |
X |
X |
| Polyamides (PA)
|
X
|
|
X |
X |
X |
| Polychlorovinyls (PVC, PVDC)
|
|
|
|
X |
X |
| Polyesters (PE)
|
|
|
X |
X |
|
| Polyolefins (PO)
|
X |
|
X |
X |
|
| Polyethylene derivatives |
|
|
X |
X |
|
| Polypropylene derivatives |
|
|
X |
X |
|
| Polyurethane (PU)
|
X |
|
X |
X |
X |
| Isocyanates |
|
|
X |
X |
|
| PU prepolymers |
X |
|
X |
X |
X |
| PU dispersions |
|
|
X |
|
|
| Polyols |
X |
|
X |
X |
|
| Thermoplastic PU |
|
|
X |
X |
|
| Polyvinyl copolymer emulsions (PVAc) |
|
|
|
X |
X |
| Styrene block copolymers (SBCs)
|
|
|
X |
X |
X |
| Styrene copolymers (SBR)
|
X |
|
X |
X |
X |
| Sulfone based
|
|
|
X |
X |
|
| Synthetic rubber
|
X |
X |
X |
X |
X |
| Chloroprene (CR) and chlorinated rubbers |
X |
|
X |
X |
X |
Antioxidant Families Used with Base Polymers in Adhesive Formulations
Antioxidants should be relatively soluble in the polymeric matrix. If the solubility limit of the antioxidant in the polymer is exceeded, exudation or migration of the antioxidant out of the formulation will occur.
Migration of the antioxidant can leads to performance issues, such as:
- Diminish adhesion by providing a weak boundary layer and
- Create surface tackiness to prevent unwinding of rolled tapes and labels.
Efficiency and Concentration
Antioxidants are used in small concentrations. The concentration will depend on the type polymer and antioxidant used and the short- and long-term exposure temperatures expected of the polymer.
Antioxidants are generally used in concentrations of about 0.05 to 0.10% as processing stabilizers and 0.10 to 0.50% as long term stabilizers.
Higher concentrations of antioxidants are used to stabilize polymers such as natural rubber.
Saturated polymers have greater oxidative stability and require relatively low concentrations of stabilizers. The specific concentration is generally determined by trial and error, but the technical literature and antioxidant supplier can be of great assistance.
Antioxidants are generally added to each raw material component of an adhesive formulation that is prone to oxidation, and in most cases they are also added to the final adhesive formulation.Low concentrations of antioxidants (< 0.01%) are added to the raw material after synthesis to protect the polymer during shipment and storage. Antioxidants applied by the formulator will not undo any oxidative damage that might be caused to the raw materials via storage, processing, etc.
The overall efficiency of antioxidants in polymers is determined by its:
- Chemical reactivity
- Physical loss during processing & service life
- Minimal effective concentration
In most cases, loss of the stabilizer by physical processes (evaporation, leaching) is more significant than the loss by chemical consumption.
The minimal effective concentration of an antioxidant is dependent on its ability to reach the polymer’s attacked sites by diffusing through the polymer.
- For this to effectively happen, the antioxidant must be compatible and soluble in the polymer.
- It must also have a low degree of volatility so that minimal antioxidant is lost during processing.
- The efficiency of an antioxidant during its service life is dependent on the above factors along with its resistance to extraction into the environment (migration).
The amine antioxidants are generally more powerful than the hindered phenols. This is due to a cyclic process which the amine antioxidant undergoes in which a nitroxyl radical is regenerated and consumes more radicals. The concentration level of phenols added to plastics is generally between 250 and 3000 ppm, depending on the character and expected lifetime of the polymer substrate. The concentration level of amines and phosphites is slightly less.
A mixture of antioxidants that function by different mechanisms might be synergistic and provide a higher degree of protection than the sum of the stabilizing activities of each component. The most frequently used synergistic mixtures are combinations of radical scavengers (primary antioxidants) and hydroperoxide decomposers (secondary antioxidants).
The table below illustrates the melt stabilizing efficiency of single antioxidants and blends for mixing polypropylene at 190°C. The melt stability is measured as reduction in melt flow index measured at 230°C.
| Antioxidant |
Trade Name
(Supplier) |
Concentration by Weight,
% |
Melt Flow Index,
g/10 min |
| Control (no antioxidant) |
– |
0 |
11.7 |
| Tetrakis (methylene-3-(3,5-di-tert-butyl-4-hydroxyphenyl) propionate) methane |
|
0.05 |
5.9 |
| 0.10 |
4.6 |
| 0.20 |
3.9 |
| Octadecyl-3-(3,5-di-tert-butyl-4-hydroxyphenyl) propionate |
Irganox® 1076
(BASF) |
0.20 |
3.7 |
| 2,6-di-t-butyl-p-cresol (butylated hydroxytoluene) |
BHT (Various) |
0.20 |
3.5 |
| Tris(2,4-di-tert-butylphenyl) phosphite |
Irgafos® 168
(BASF) |
0.05 |
7.7 |
| 0.10 |
7.2 |
| Thioester heat stabilizer blend |
Irganox® PS 800
(BASF) |
0.10 |
8.4 |
| Alpha-tocopherol |
(Various) |
0.05 |
3.8 |
| 0.10 |
3.6 |
| Blend of primary and secondary antioxidants |
Irganox® 1010 and Irgafos® 168 |
0.05 + 0.05 |
4.3 |
| Irganox® 1010 and PS 800 |
0.05 + 0.10 |
4.9 |
Melt Stabilizing Efficiency of Antioxidants in Polypropylene (processed in an internal mixer at 190°C). Melt flow index (MFI) measured at 230°C for 2.16 kg charge.
Temperature During Compounding and Service
Antioxidants are not equally effective at all temperatures. The figure below illustrates the effective temperature ranges for selected types of antioxidants.
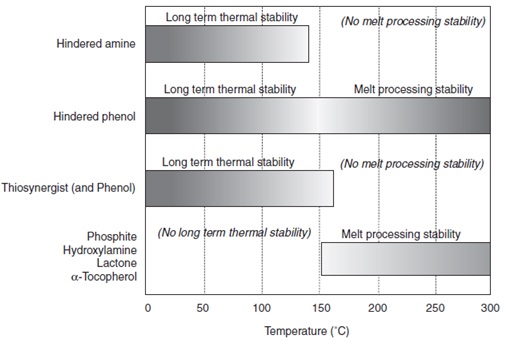 General Representation of Effective Temperature Ranges for Selected Types of Antioxidants.3
General Representation of Effective Temperature Ranges for Selected Types of Antioxidants.3
Phenolic antioxidants are useful during both processing and as long term stabilizers. Irganox® 1010 is the most well-known and generally used product in this family, and many others have been developed for specific applications.
Hindered amines (more commonly thought of as being UV stabilizers) are also useful for long-term thermal stability below the melting point of the polymer. This is due to the fact that hindered amines work by a free radical scavenging mechanism. However, hindered amines are ineffective at temperatures above 150°C. For overall thermal stability, hindered amines should always be used in combinations with an effective melt processing stabilizer.
Phosphorus, hydroxylamine, and lactone-based compounds are most effective at elevated temperatures, and they are commonly used as melt process stabilizers during processing and compounding. They are not effective long-term stabilizers. Often these antioxidants are used in stabilizer systems with phenolics to provide protection during melt processing and lessen the workload of the phenolic component.
Physical Property Deterioration
All polymers are subject to degradation on exposure to oxidative environments. These degradative factors will cause substantial changes in properties such as shown in the table below.
| Type of Degradation |
Initiating / Accelerating Factors |
Degradation Causes |
Resulting Change in Properties |
| Storage aging |
Surrounding environmental conditions |
Oxygen, light, heat, humidity |
Loss of elasticity and tensile strength |
| Aging due to heat |
Heat |
Oxygen |
Loss of elasticity and tensile strength |
| Aging due to light and weathering |
Light, UV, heat, humidity, surrounding environmental conditions |
Oxygen |
Formation of crazed surfaces, loss of elasticity and tensile strength |
| Contribution of soluble metal ions to catalyze oxidation |
Cu, Fe, Mn, Co, and Ni ions due to catalysts and other components |
Oxygen |
Rapid loss in elasticity and tensile strength |
| Flex-fatigue cracking |
Intermittent mechanical strains |
Oxygen, ozone, flaws |
Appearance of surface cracking patterns |
| Ozone cracking |
Continuous / intermittent strains |
Ozone |
Extensive cracking at right angles to force causing strain |
Type of Oxidative Degradation, Initiating Factors, Causes,
and Resulting Changes in Properties
Therefore, the antioxidant should be selected to minimize changes to bulk properties such as tensile strength, elongation at break, and impact strength.
- Changes in these properties will also affect the following adhesive properties, such as:
- Shear & peel strength
- Tack
- Toughness
- Flexibility, and
- Resistance to low temperature environments
- Processing property changes due to oxidation include:
-
Skin formation,
- Gel formation, and
- Changes in viscosity
- Aesthetic changes include:
- Discoloration and
- Loss of clarity
Information on selection of antioxidants relative to these changes is difficult to find due to the many different base polymers, antioxidants, and processing conditions that are possible. The formulator usually must rely on trial and error in specific formulations.
Volatility
Most additives are melt compounded into the polymer after the polymerization stage. The melt compounding and downstream processing steps represent significant heat histories. Storage of the product can also be quite warm in certain climates. It is important that the antioxidant, or the products resulting from its reaction that may also provide stability, not volatilize from the polymer.
Volatility of antioxidant is related to both the molecular weight and the type of molecule. Antioxidants with higher molecular weights generally have a lower degree of volatility and are more resistant to extraction or migration during service life. For example, Irganox® 1010 is exceptional among hindered phenolic antioxidants. It has one of the highest molecular weights in its class and is non-staining and non-discoloring. The type of molecule, however, has a greater effect than the molecular weight. For example, the hindered phenols have relatively high volatility in comparison with main antioxidants of the same approximate molecular weight.
The
table below shows the significant effect that different antioxidants can have on volatility in natural rubber when aged at 100°C.
|
Antioxidant
|
Trade Name (Supplier)
|
Loss on Heating at 100°C, weight %
|
|
4 Days
|
8 Days
|
12 Days
|
|
N,N’-Bis (1,4-dimethylpentyl)-p-phenylenediamine
|
Santoflex 77PD (Eastman)
|
7.50
|
13.20
|
17.00
|
|
N-phenyl-N’ isopropyl-p-phenylenediamine
|
Antioxidant IPPD (Hainan Huarong Chemical)
|
4.25
|
6.40
|
9.00
|
|
N-(1,3-Dimethylbutyl)-N'-phenyl-p-phenylenediamine
|
Santoflex 6PPD (Eastman)
|
2.50
|
4.50
|
6.20
|
|
Polymerized 2,2,4-Trimethyl-1,2-dihydroquinoline
|
Sovchem TMQ (Sovereign Chemical)
|
0.15
|
0.20
|
0.20
|
Volatility of Selected Antioxidants at 100°C.4
Migration
Migration of antioxidants out of the polymer during service life is especially important in certain applications such as food packaging where the antioxidants can affect taste and odor and adhesives where the antioxidants can form a weak boundary layer to reduce adhesive strength.
Similar to volatility, migration will depend strongly on the type of antioxidant and its molecular weight. However, it will also be affected by the type of base polymer, the environment to which the stabilized product is exposed, and the temperature.
In a study of high temperature migration of Irganox® 1010 and 1076 from polyolefins in water, aqueous ethanol, and corn oil at high temperatures. Goydan, et. al.5 found that the migrations varied with the square root of time, and the diffusion coefficients could be correlated with temperature in an Arrhenius fashion.
- Under comparable test conditions, antioxidant migrations were largest from PP for aqueous simulants, but for non-aqueous simulants the highest losses were from LDPE.
- In both instances lowest losses were from HDPE.
- In most instances there was little difference between the migration behaviors of the two antioxidants tested.
Form
Antioxidants are commercially available in several forms – solids of various
forms, liquids, and emulsions or dispersions in water. The following table
provides examples of the forms available for the most common families of
antioxidants.
Several Available forms of Antioxidants
Antioxidants in the form of liquids and pellets are challenging the powder form. Advantages include low dusting, improved safety, and lower cost. However, some polymer raw material manufacturers need the additives as fine powders in order to achieve good premixing with their reactor product before melt compounding into the traditional pellet form.
Color
Upon oxidation phenolic antioxidants impart much less color than aromatic
amine antioxidants and are non-discoloring and non-staining. AO-1010 especially
is non-staining, non-discoloring, and effective in non-black, colorable compounds.
Phosphorous compounds are used most frequently in combination with hindered phenols for a broad range of applications in rubber and polymers. They are also able to suppress color development caused by oxidation of the substrate and the phenolic antioxidant. Unlike phenols and secondary aromatic amines, phosphorus-based stabilizers generally do not develop colored oxidation products.
Hindered amines are extremely effective in protecting polyolefins and other polymeric materials against photodegradation. They usually are classified as light stabilizers rather than antioxidants.
It is important that antioxidants do not provide unwanted color due to the transformation chemistries associated with preventing oxidation. Some antioxidants are prone to forming color by their very nature,
while other antioxidants discolor only when they have been over oxidized. In these cases, pigments can be used to mask the subtle changes in the base color of the polymer.
Toxicity and Regulatory Approvals
The required approvals for the specific application (e.g., for food packaging or medical applications) needs to be considered when selecting an antioxidant. Safety is generally assessed by subjecting the antioxidant to a series of animal toxicity tests similar to those used to test food packaging or medical polymer additives.
Granulated and liquid forms of antioxidants are receiving greater acceptance to minimize the inhalation of dust.
A number of antioxidants have been regulated by approval of government authorities as indirect additives for polymers used in food contact applications. Acceptance is determined by:
- Subchronic or chronic toxicity and
- The concentration expected in the diet, based on the amount of the additive extracted from the polymer by solvents that simulate food in their extractive effects.
Broad food contact regulation is increasingly a requirement for the successful introduction of a new antioxidant.
Cost
It may seem apparent that increasingly higher levels of antioxidant will provide greater protection. However, this is often not true. Unfortunately, these are relatively expensive materials and excessive concentrations could cause migration and degrading effects on other properties. Their use, therefore, requires a balance between the desired prevention level and the cost of the raw material.
A number of the world's leading producers of polymer antioxidants have introduced price increases in recent years. This is generally attributed to increasing costs of raw materials, energy, packaging and freight.
Without the price increases the companies say they will be unable to ensure product availability or invest in innovation and increased capacity to meet growing demand for antioxidants. However, price increases are somewhat limited by the shift of antioxidant production to Asia.
Tests to Determine Efficiency of Selected Antioxidants
Tests to Determine Efficiency of Selected Antioxidants
There are a variety of test methods available for selecting and testing the right antioxidant for an adhesive formulation and specific application. These test methods generally monitor the effectiveness of the antioxidant package by change in physical property on aging.
Regular determinations of color, mechanical properties, or other parameters are used to follow the changes of the specimen. Analytical tests such as oxygen index time by differential scanning calorimetry (DSC) can also be used to determine the effectiveness of various antioxidants.
Suitable antioxidants for plastics have to be selected according to two important phases of their life cycle:
- Stabilization of the resin during high temperature processing, and
- Stabilization during its service life
Analytical Test Methods
Oxygen induction time is a sensitive measure of the efficiency of anti-oxidative additives within the polymer. Oxidative induction experiments can be made with differential scanning calorimetry (DSC) equipment and they are used to quickly assess the relative efficiency of various antioxidant systems6.
The experiments are typically conducted under severe testing conditions, typically at temperatures above the melting point of the polymer. This method is not useful for predicting long term thermal stability.
For the oxygen induction test, a sample is heated under a nitrogen atmosphere, typically to 200°C. Oxygen is then introduced to the sample cell. After a certain time, the exothermal oxidation of the sample starts, and this is noted as oxidation induction time.
The figure below illustrates the results of an oxygen induction time measurement using differential scanning calorimetry. The method is standardized in ASTM D 3895 and DIN EN 728 for polyolefins. The better the performance of the antioxidant, the longer is the oxygen induction time.
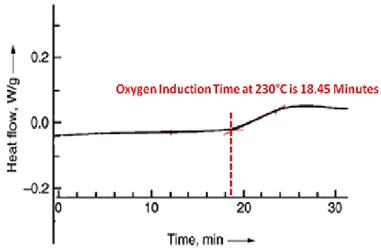 Differential scanning calorimetry (DSC) diagram to determine oxygen induction time 7
Differential scanning calorimetry (DSC) diagram to determine oxygen induction time 7
High Temperature Processing Test Methods
During any high temperature processing step, the polymer is exposed to shear and high thermal stress within a short period. This can typically be simulated by aging in the melt form (as a hot-melt tank). Or if the adhesive is extruded into a film or onto a carrier, the aging process can be accelerated with multiple pass extrusions in the laboratory where the extrusion step is repeated at least five to ten times.
After hours of aging in the melt tank or after each extrusion step properties such as melt viscosity and color can be measured. GPC, IR, and other analytical methods can be used to follow the polymer degradation in more detail.
Long-term Aging Test Methods
In oven aging tests to monitor service life at temperatures below the melt temperature, one can conduct tests at three or more temperatures and then extrapolate the oxidative degradation on physical properties to other temperatures using an Arrhenius plot.
Find Suitable Antioxidant Grade
View a wide range of antioxidant grades available in the market today, analyze technical data of each product, get technical assistance or request samples.
References
- Fay, J.J. and Fagouri, C., “Antioxidants, UV Stabilizers, and Other Functional Polymer Additives for Hot Melt Adhesives”, 2003 TAPPI Polymers, Laminations, and Coatings Hot Melt Symposium, Orlando, FL.
- Earhart, N.J., et. al., “Thermal Stabilization of Adhesives”, Chapter 19 in Handbook of Adhesives Technology, 2nd ed., Pizzi, A. and Mittal, K. eds., Springer Publishing, New York, 2003.
- Encyclopedia of Polymer Science and Technology, John Wiley and Sons, Inc., New York, 2014, Vol. 5, p. 179.
- Antioxidants and Antidegradants, Arvind Mafatlal Group, Nocil Limited, http://www.nocil.com/Downloadfile/ETechnicalNote-Antioxidants-Dec2010.pdf, accessed May 9, 2016.
- Goydan, R., et. al.,” High-Temperature Migration of Antioxidants from Polyolefins”, Food Additive Contamination, Vol. 7, No. 3, pp. 323-337, 1990.
- Determination of the Oxidation Induction Time or Temperature, Netzsch, https://www.netzsch-thermal-analysis.com/us/materials-applications/polymers/determination-of-the-oxidation-induction-time-or-temperature-oit-and-oot/.
- Ullmann’s Encyclopedia of Industrial Chemistry, “Antioxidants”, John Wiley and Sons, New York, 2014, p. 30.