Need for Flame Retardant Adhesives
Need for Flame Retardant Adhesives
Great attention is being paid to how materials perform in combating the effects of fire. This is partly due to several terrifying incidents associated with the outbreak of fire in public facilities that have led to significant damage and personal injury.
In addition to a greater level of awareness regarding fire safety, several paths have been identified to mitigate the destruction caused when fire erupts. These include:
- Improved design and
- The use of fire resisting materials
The use of adhesives is intimately connected with both.
In certain applications the adhesive may require fire resistant properties through the addition of a flame retardant. These properties may be directed at the bulk adhesive itself or at the entire bonded joint.
An example of the latter case is flexible circuitry used in electronic and transportation industries. Here the laminating adhesive may be asked to provide sufficient flame retardance to effect the final product (polyester film bonded to copper foil), and in this case the entire joint is tested, not only the adhesive.
A flame resistant adhesive formulation may be required to meet the following criteria:
- Resist burning
- Retain joint integrity at high temperatures
- Produce minimal smoke, and
- Release negligible toxic material when heated beyond its decomposition temperature
In addition, a flame retardant must not affect processing methods or the normal service performance of the adhesive (adhesion, durability, chemical resistance, etc.)
Let's explore how to prevent the four stages of fire by studying about the functions provided by flame retardants
in detail...
Functioning of Flame Retardants
Functioning of Flame Retardants
Flame retardants inhibit or delay the spread of fire by suppressing the chemical reactions in the flame or by the formation of a protective layer on the surface of a material.
They may be mixed with the base material (additive flame retardants) or chemically bonded to it (reactive flame retardants). Mineral flame retardants are typically additive while organic compounds can be either reactive or additive.
Designing Fire-Retardant Adhesive
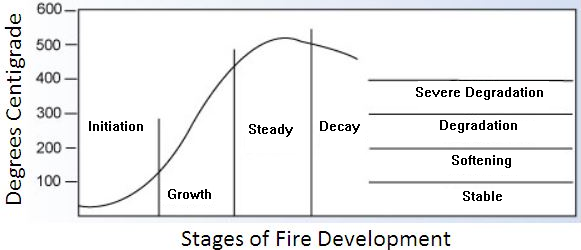
A fire effectively has four stages:
- Initiation
- Growth
- Steady State, and
- Decay
 Comparison of the Degradation Temperatures of
a Typical Thermoset Adhesive
Comparison of the Degradation Temperatures of
a Typical Thermoset Adhesive
With Those Reached in Various Stages of a Fire
Each state has a corresponding degradation temperature as shown in Figure. In designing a fire-retardant adhesive, formulators must put their efforts on delivering temperature resistance on the right fire stage for the application:
- In electronic manufacturing, for example, an adhesive must suppress any tendency of the electronic component to catch fire - or initiate - if there is a fault-induced rise in temperature.
- For bonding tiles or panels, adhesives need to resist detachment in the growth and steady state stages, even when in direct contact with the flame.
- They must also minimize toxic gases and smoke emitted. Load-bearing structures are likely to experience all four stages of the fire.
Limiting Combustion Cycle
To limit the combustion cycle, one or several of the processes contributing to fire must be removed by either:
- Elimination of the volatile fuel, as by cooling
- Production of a thermal barrier, as by charring, thus eliminating fuel by reducing heat transfer, or
- Quenching the chain reactions in the flame, as by adding suitable radical scavengers
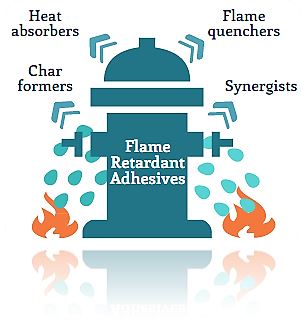
Flame retardant additives do this by acting chemically and/or physically in the condensed (solid) phase or in the gas phase by providing one of the following functions:
- Char formers : Usually phosphorus compounds, which remove the carbon fuel source and provide an insulation layer against the fire’s heat. There are two char forming mechanisms:
- Redirection of the chemical reactions involved in decomposition in favor of reactions yielding carbon rather than CO or CO2 and
- Formation of a surface layer of protective char
- Heat absorbers : Usually metal hydrates, such as aluminum trihydrate or magnesium hydroxide, which remove heat by the evaporation of water from the flame retardant’s structure.
- Flame quenchers : Usually bromine- or chlorine-based halogen systems which interfere with the reactions in a flame.
- Synergists : Usually antimony compounds, which enhance performance of the flame quencher.
Significance of Flame Retardants in Fire Protection
Flame retardants are an important part of fire protection as they not only reduce the risk of a fire starting, but also that of its propagation. This increases escape time and, thus, protects humans, property, and the environment.
There are many ways to establish an adhesive as a fire retardant. Let's
understand the classification of flame retardants in detail.
Classification of Fire Retardants
Classification of Fire Retardants
Flame or fire retardants are classified on two basis:
#1. On the Basis of Reactivity
Flame retardants for polymers can be classified as:
- Reactive - the flame retarding agent reacts chemically with the polymer to become an integral part of the molecule
- Additive - nonreactive agents that are simply blended or mixed into the compound
Additive flame retardants are more common in practice as
the choice of a reactive flame retardant is a bit complex.
The development of systems based on reactive flame retardants is more expensive for the manufacturer, who in effect has to develop novel co-polymers with the desired chemical, physical and mechanical properties, as well as the appropriate degree of flame retardance.
Reactive Flame Retardants
They become a part of the polymer either by becoming a part of the backbone or by grafting onto the backbone. Therefore, they have little influence on mechanical properties. These include tetra-bromo-biphenyl A, di-bormo-neophenyl glycol, brominated styrene, etc.
Non-reactive Flame Retardants
These are not chemically reacted onto the base polymer backbone and, therefore, may migrate out of the system during processing or service life reducing the effectiveness over time. Typical non-reactive flame retardants include chlorinated paraffins, brominated organics, phosphate ester, aluminum trihydrate, magnesium hydroxide, borates, and antimony trioxide.
#2. On the Basis of Chemical Group
Flame retardants can also be classified by the major chemical group which provides the flame retardant properties.
a. Classification on the Basis of Family
With this classification system, there are four main families of flame-retardant chemicals as shown in below.
|
Family |
Characteristics |
|
Inorganic
|
- The main inorganic flame retardants are aluminum trihydroxide, magnesium hydroxide, ammonium polyphosphate, and red phosphorus.
- This group represents about 50% by volume of the worldwide flame retardant production.
- Some of these chemicals are also used as flame retardant synergists, of which antimony trioxide is the most important.
|
|
Halogenated
|
- Halogenated products are based primarily on chlorine and bromine.
- This group represents about 25% by volume of the worldwide production.
- Synergists such as antimony oxides are frequently used with halogenated flame retardants.
|
|
Organophosphorous
|
- Organophosphorus products are primarily phosphate esters and represent about 20% by volume of the worldwide production.
- Phosphate esters, with or without halogen, are the predominant phosphorus-based flame retardant in use.
- Phosphoric acid esters provide plasticization in addition to flame retardancy.
|
|
Nitrogen
|
- Nitrogen-based flame retardants are used for a limited number of polymers.
- Nitrogen-based compounds can be employed in flame-retardant systems or form part of intumescent flame-retardant products.
- Melamine, melamine cyanurate, other melamine salts, and guanidine compounds are currently the most predominant group of nitrogen-containing flame retardants.
|
b. Classification on the Basis of Chemical Type
The major flame retardant products that are being used today in adhesive and sealant formulations and their primary applications are shown in below.
|
Types
|
Typical Products |
Typical Applications |
|
|
Available in various particle size grades and in surface treated grades
|
- Polyesters
- Epoxies
- Polypropylene and ethylene /
rubber compounds
|
|
|
Dusting and non-dusting grades are available of various particle sizes |
Works
synergistically with reactive or additive halogenated compounds, also:
- Polyethylene
- Polypropylene
- Thermoplastic and
- Unsaturated polyesters
|
|
Borate-based
|
Zinc borate,
Barium metaborates Ammonium fluoroborate Boric acid
|
- Flexible PVC
- Polyolefins
- Thermoplastic and unsaturated
polyesters
- Epoxies
- Polyamides
- Urethanes
|
|
|
Aromatic bromines (e.g.
decarbromophenyl oxide ether, tetrabromo-bisphenol A, pentabromodiphenyl
oxide) Aliphatic bromines Ionic bromines Benzene-ethyltribromo
derivatives Polypentabromobenzyl acrylate
|
Work
synergistically, also:
- Polyolefins
- Polyesters: thermosets and
thermoplastics
- Polyurethanes
- Epoxies
- Polyamides
|
|
|
Chlorinated paraffins, Tris (dichloropropyl)
phosphate, methyl pentachloro stearate, and other chlorinated phosphates Cycloaliphatic chlorine Chlorendic anhydride |
Reactive intermediate in making polyester and epoxy flame retardant
resins, also:
- LDPE
- PVC
- Polyurethane
- Polypropylene
- Polyamide
|
|
Magnesium-based
|
Magnesium hydroxide, Magnesium carbonate
|
Synergist with alumina trihydrate for smoke reduction, also:
- Polyesters
- Epoxies
- Polypropylene and
- Ethylene /
rubber compounds
|
|
|
Pure melamine, Melamine derivatives (salts
and inorganic acids), melamine homologues
|
- Polyurethane
- Polyamide
- Polyolefins
- Thermoplastic
- Polyesters
|
|
|
Phosphate esters and others
(halogenated and non-halogenated)
|
- Polyurethane
- Polyesters
- PVC
- Celullosics
- Polyethylene
- Polypropylene
- Ethylene / propylene
copolymers
|
|
Silicone
|
Polydimethylsiloxane
|
Works
by producing a char surface with:
- Polyolefin and polyolefin
blends
- EVA
- Polyurethane
|
Selection Criteria for Flame Retardants
Selection Criteria for Flame Retardants
The criteria in selecting and using the right flame retardant system for a specific adhesive formulation can be broad and somewhat complex.
Care must also be taken with regard to choosing the proper test method and determining the parameters of the test. The materials chosen to perform the function of flame retardation must not interfere with the final product’s performance.
The major problem with incorporating flame retardants in adhesives is that very often a significant amount is required, and they interfere with the other properties of the adhesive and contribute to the cost. Consultation with the flame retardant suppliers is generally necessary to optimize the selection process.
The key criteria used to select a particular flame retardant are:
Fire Retardancy
Identification of the flammability test requirements is the initial criterion for selection of the appropriate flame retardant. Flame-retardant selection is affected by the test method to be used to assess flame retardancy.
Some tests can be passed with relatively low levels of flame retardants, while high levels of very powerful flame retardants are needed to pass other tests. Test requirements and standards are usually unique to an industry such as electrical / electronic or building and construction.
Most test requirements apply to the completely assembled and manufactured product and not simply to the adhesive material.
Several fire retardancy characteristics for major chemical families of flame retardant additives are provided. These characteristics are:
- Acting site (gas or solid phase)
- Mode of action (chemical or physical)
- Relative efficiency
|
Characteristic
|
Chemical Family of Flame Retardant
|
| Inorganic
|
Hydrogenated
|
Organo-
phosphorus
|
Nitrogen
|
Acting site
|
Condensed (solid) /
gas phase
|
Gas phase
|
Condensed (solid) / gas phase
|
Condensed (solid) / gas phase
|
| Mode of action |
Physical
|
Chemical
|
Chemical /
physical
|
Chemical /
physical
|
| Flame retarding efficiency |
Poor
|
Good
|
Good
|
Good
|
Characteristics of Major Chemical Families of Flame Retardant Additives
Efficiency/Cost
The relationship between fire retardant efficiency and cost is an essential consideration in the selection of a flame retardant.
The greater the flame retardant efficiency of a product for a particular resin, the less will be needed. The amount to be used also depends on the application and specification.
The performance / cost ratio of several common flame retardants are shown below.
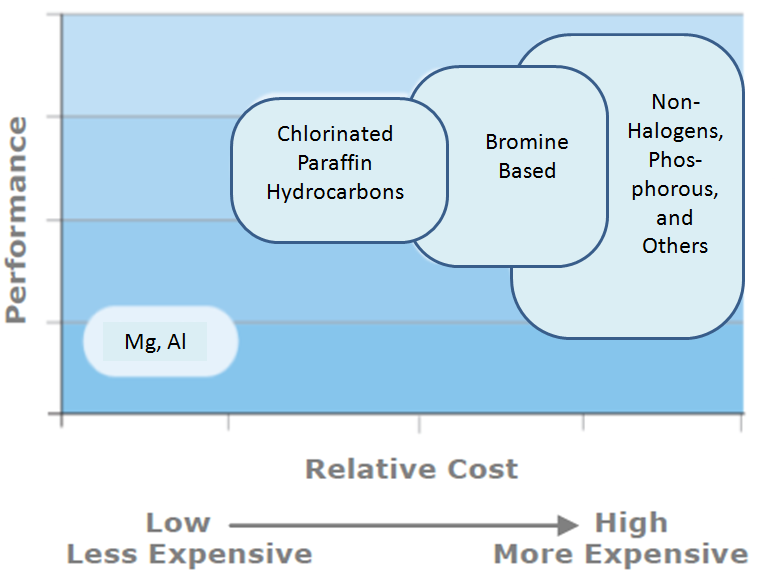 Flame Retardancy Performance and Relative Cost of
Common Flame Retardants
Flame Retardancy Performance and Relative Cost of
Common Flame Retardants
(Dover Chemical
Corporation, January 2016)
It can be concluded from the figure that:
- Aluminum trihydrate - It is the lowest cost additive and is used mostly in polymers processed at low temperatures:
- Epoxy
- Unsaturated polyesters
- Polyethylene
- PVC
However, high loadings are required and this can affect the physical properties of the polymer.
- Chlorinated paraffins - These paraffins are low cost and can be applied in all polymers processed under 240°C.
- Bromine based - These flame retardants are more effective on a weight basis. However based on cost performance, chlorinated paraffins are more effective than aromatic bromine flame retardants.
Smoke and Combustion Products
When fire occurs in a confined space, such as a home or airplane passenger compartment, often the gases generated due to the combustion process provides the most serious threat. As a result, considerable attention has been given to the generation of smoke and toxic combustion products.
Given free access to oxygen, polymers like polyethylene that are based entirely on carbon and hydrogen will burn to give a mixture of water vapor and CO2, but form toxic CO if without enough oxygen. Polymeric materials which contain the elements chlorine, nitrogen and sulfur will always burn to produce noxious and toxic materials whether there is an adequate oxygen supply or not.
The table below provides characteristics for major chemical families of flame retardant additives. These characteristics are:
- Acting site (gas or solid phase)
- Mode of action (chemical or physical)
- Side effects (smoke and decomposition products)
|
Characteristic
|
Chemical Family of Flame Retardant
|
| Inorganic |
Hydrogenated |
Organo-
phosphorus
|
Nitrogen
|
Acting site
|
Condensed (solid) /
gas phase
|
Gas phase
|
Condensed (solid) / gas phase
|
Condensed (solid) / gas phase
|
| Mode of action |
Physical
|
Chemical
|
Chemical /
physical
|
Chemical /
physical
|
| Minimization of smoke and toxic combustion products
|
Good
|
Poor
|
Good
|
Good |
Characteristics of Major Chemical Families of Flame Retardant Additives
Ease of Compounding
Flame retardant additives may adversely affect the processing characteristics of polymers. Changes occurring in the viscosity of liquid systems or in the flow of polymers that are melted during processing can cause problems.
Non-melting flame retardants can make the base polymer more difficult to process. However, melt bendable and plasticizing additives such as phosphoric acid esters may be desirable. These can act as processing aids, reduce internal stress in the bond line, and generally provide the adhesive with higher flexibility and impact strength.
Flame retardants can be supplied in various forms to aid in processing of the final adhesive.
The table below shows the available forms of some common flame retardant additives:
Various Forms of Common Flame Retardants
Compatibility
Polymer compatibility indicates how well the flame retardants interact with the base polymer with which it is mixed. Issues related to solubility, chemical resistance, or reactivity with other formulation components may prevent the use of an otherwise desirable flame retardant.
Significant alteration of the rate of reaction of thermoset polymers or the speed and degree of crystallization of thermoplastic polymers may also result from the use of some flame retardants.
The flame retardant should be chemically compatible with the base polymer as well as mix and disperse well and even dissolve in the polymer if possible.
Typical
Base
Polymers
|
Type of Flame Retardants |
|
Alumina Trihydrate |
Antimony Oxide |
Borate-based |
Bromine-based |
Chlorine-based |
Magnesium-based |
Melamine-based |
Phosphorous-based |
Silicone |
|
Acrylic
&
Acrylic Co-polymers |
|
|
|
|
|
|
√ |
√ |
|
|
Epoxies |
√ |
√ |
√
|
√
|
√
|
√ |
√ |
√ |
|
|
Ethylene Coter-polymer Solids (EVA, EMA)
|
|
|
|
|
√ |
|
√ |
√ |
√ |
|
Natural Rubber |
|
|
|
|
|
|
√ |
|
|
|
Polyamides |
|
|
√ |
√
|
√ |
|
√ |
√ |
|
|
Poly-chloro-vinyls (PVC, PVDC) |
|
√ |
√
|
√
|
|
|
|
√
|
|
|
Polyesters |
√ |
√ |
√ |
√ |
√ |
√
|
√ |
√ |
|
|
Polyolefins |
|
√ |
√ |
√
|
√ |
|
√ |
√ |
√ |
|
Polyurethanes |
|
√
|
√ |
√
|
|
|
√
|
√ |
√ |
|
Silicones |
|
|
|
|
|
|
√
|
|
√ |
|
Synthetic Rubbers |
|
|
√ |
|
|
√
|
√
|
√ |
|
Flame Retardants Suggested for Use with Various Base Polymers
Incompatible flame retardants are more likely to migrate out of the formulation during processing or service aging. Higher molecular weight polymeric additives are a response to this problem and many are available. Reactive flame retardants become a permanent part of the polymer chain and cannot migrate or volatilize.
Adequate Processing Temperature Stability
For the formulator, the flame retardant must be sufficiently stable to survive the processing temperatures. The temperatures routinely used to process many polymers severely restrict the number of flame retardants suitable for incorporation.
Yet it must not be too stable to function as an effective flame retardant when in service. Typically, thermal gravimetric analysis (TGA) is used to evaluate the decomposition or volatilization temperatures of flame retardants and to aid in the selection of the most cost effective materials.
For example: The figure below compares the decomposition characteristics of alumina trihydrate and magnesium hydroxide:
- Alumina trihydrate decomposes at about 220°C
- While magnesium hydroxide decomposes at about 330°C, thus having a higher thermal stability allowing for a wider window for compounding processing.
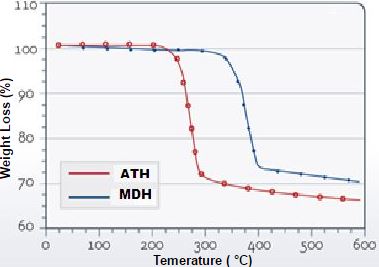 Decomposition Temperatures of Alumina Trihydrate (ATH) and
Magnesium Hydroxide (MDH)
Decomposition Temperatures of Alumina Trihydrate (ATH) and
Magnesium Hydroxide (MDH)
Measured by Thermal Gravimetric Analysis
(Hubber Fire Retardant Additive Solutions, J.M. Hubber Corporation, 2011)
Effect on Properties
A flame retardant must be chosen for a specific polymer to minimize the degradative effect that it may have on mechanical and thermal properties. Since flame retardants are frequently used at high levels, they often have a dramatic effect on the basic mechanical properties of adhesive systems in which they are used.
Some common problems include reduction of:
- Strength
- Rigidity
- Toughness
- Heat resistance
When flame retardants are added to polymers, following changes occur:
- Physical properties (density, hardness, melting point, glass transition temperatures, and thermal expansion) and appearance (color, transparency) often change significantly.
- Electrical properties (resistance, dielectric strength, loss factor) are frequently altered.
- Adhesive’s durability due to factors such as oxidation, UV radiation, and high temperatures may be reduced.
The chemical properties of a flame retardant are also often of great importance in the selection process. Resistance to exposure to water, solvents, acids, bases, oils or other substances may be a requirement for use.
Environmental Regulations
The environmental demands placed on today’s flame retardants have changed considerably over the past and play a decisive role in selection. There are some environmental concerns that have developed over recent years regarding flame retardants. The following could influence their selection for a specific application:
- Findings of certain brominated flame retardants in the environment
- Animal and plant life, and in humans
- Some concern about certain phosphate esters in indoor air
- Flame retardant persistence in the environment, bio-accumulation, toxicity, etc.
Although bromine and chlorine-containing flame retardants are still used in some products, the need for new alternatives is being driven by a convergence of:
- Policy
- Standards and
- Pressure from environmental groups
Environment regulations tend to be industry and region-specific
A large group of the studied flame retardants has been found to have a good environmental and health profile. These are:
- Ammonium polyphosphate
- Aluminum diethylphosphinate
- Aluminum hydroxide
- Magnesium hydroxide
- Melamine polyphosphate
- Dihydrooxaphosphaphenanthrene
- Zinc stannate
- Zinc hydroxstannate
Overall, they were found to have a much lower tendency to bioaccumulate than for studied brominated flame retardants.
Standards and Test Methods for Flame Retardants
Standards and Test Methods for Flame Retardants
Standards related to fire testing are aimed at determining the performance of a material with respect to flame, smoke, and toxicity (FST). Several tests have been widely used to determine the resistance of materials to these conditions.
Selected Tests for Flame Retardants
Resistance to Burning
- ASTM D635: “Rate of
Burning of Plastics”
- ASTM E162:“Flammability of Plastic Materials”
- UL 94: “Flammability of
Plastic Materials”
- ISO 5657: “Ignitability of
Building Products”
- BS 6853: “Flame
Propagation”
- FAR 25.853: “Airworthiness
Standard – Compartment Interiors”
- NF T 51-071: “Oxygen
Index”
- NF C 20-455: “Glow Wire
Test”
- DIN 53438: “Flame Propagation”
|
Resistance to High Temperatures
- BS 476 Part No. 7:
“Surface Spread of Flame – Building Materials”
- DIN 4172: “Fire Behaviors
of Building Materials”
- ASTM E648: “Floor Coverings – Radiant Panel”
|
Toxicity
- SMP 800C: “Toxicity
Testing”
- BS 6853: “Smoke Emission”
- NF X 70-100: “Toxicity
Testing”
- ATS 1000.01: “Smoke Density”
|
Smoke Generation
- BS 6401: “Specific Optical
Density of Smoke”
- BS 6853: “Smoke Emission”
- NES 711: “Smoke Index of
Products of Combustion”
- ASTM D2843: “Smoke Density
from Burning Plastics”
- ISO CD5659: “Specific
Optical Density – Smoke Generation”
- ATS 1000.01: “Smoke
Density”
- DIN 54837: “Smoke Generation”
|
Testing Resistance to Burning
In most tests that measure the resistance to burning, suitable adhesives are those that do not continue to burn for any significant period after removal of the source of ignition. In these tests the cured adhesive sample may be subjected to ignition independent of any adherend (the adhesive is tested as a free film).
Although this approach does not simulate practical reality, it does provide useful data on the relative resistance of the adhesive to burning.
Sample structures with both adhesive and adherend can also be tested. These results may be more representative of the performance of the adhesive in an actual fire since the contribution provided by the adherend could be either positive or negative.
UL-94 Vertical Burning Test
It provides a preliminary assessment of relative flammability and dripping for polymers used in electrical equipment, electronic devices, appliances, and other applications. It addresses such end-use characteristics of ignition, burn rate, flame spread, fuel contribution, intensity of burning, and products of combustion.
Working and Set Up - In this test a film or coated substrate sample is mounted vertically in a draft free enclosure. A burner is placed beneath the sample for 10 seconds and the duration of flaming is timed. Any dripping that ignites surgical cotton placed 12 inches below the sample is noted.
The test has several classifications:
- 94 V-0: No specimen has flaming combustion for more than 10 seconds after ignition. Specimens do not burn up to the holding clamp, drip and ignite the cotton, or have glowing combustion persisting for 30 seconds after removal of the test flame.
- 94 V-1: No specimen shall have flaming combustion for over 30 seconds after each ignition. Specimens do not burn up to the holding clamp, drip and ignite the cotton, or have an afterglow of more than 60 seconds.
- 94 V-2: This involves the same criteria as V-1, except that the specimens are allowed to drip and ignite the cotton below the specimen.
Other Strategies for Measuring Burning Resistance
Another method for measuring the burning resistance of a material is to measure the limiting oxygen index (LOI). The LOI is the minimum concentration of oxygen expressed as a volume percent of mixture of oxygen and nitrogen that just supports flaming combustion of a material initially at room temperature.
The resistance of an adhesive to high temperatures in the case of a fire needs special consideration aside from the flame, smoke, and toxicity effects. Often the substrate will protect the adhesive from a fire. However, if the adhesive loosens or degrades due to the temperature of the fire, the joint can fail causing separation of the substrate and the adhesive. If this happens, the adhesive itself becomes exposed together with the secondary substrate. These fresh surfaces then can contribute further to the fire.
The NIST smoke density chamber (ASTM D2843, BS 6401) is used widely in all industrial sectors for the determination of smoke generated by solid materials and assemblies mounted in the vertical position within a closed chamber. Smoke density is measured optically.
When an adhesive is sandwiched between two substrates, the fire resistance and thermal conductivity of the substrates control the decomposition and smoke emission of the adhesive
In smoke density tests, adhesives can be tested alone as a free coating to impose a worst case condition.
Find Suitable Flame Retardant Grade
View a wide range of flame retardant grades available in the market today, analyze technical data of each product, get technical assistance or request samples.