Problems Encountered Due to Foaming
Problems Encountered Due to Foaming
Foaming is a problem that plagues many adhesive applications. Emulsion adhesives are especially prone to the troubles and expensive consequences created by foaming.
When any emulsion is moving in contact with air, there is a risk of foam formation.
Therefore, foam is generally encountered when mixing the adhesive or during high speed coating processes.
Foam is often an undesirable consequence of the water-borne adhesive polymerization, compounding, or conversion processes. Foam can also develop during other stages of the adhesive’s life cycle such as during filling or packaging, transportation, and coating or application.
Foaming can deteriorate adhesive system by:
- Reducing Adhesion and Aesthetics:
Foaming can lead to:
- Process inefficiency
- Overflow in tanks
- Instability of the adhesive emulsion, and
- Poor substrate coating
In applying an adhesive coating, for example, foaming problems can result in several types of defects, most notably:
- Cratering
- Pin-holing (fisheyes), and
- Dewetting
These not only reduce the aesthetics of the adhesive coating, but also can significantly reduce the adhesion properties of the finished product.
- Increasing Viscosity of the System:
The formation of bubbles or foam on a thin adhesive coating requires special consideration. In a water-borne adhesive coating applied to a substrate, as the water in the formulation evaporates at the surface it causes an increase in viscosity.
This viscosity increase prevents the smaller bubbles within the coating from rising to the surface, and also inhibits the possibility of the liquid from flowing back into the space originally occupied by the bubble. This situation causes craters to form in the coating. The smaller bubbles that remain in the coating because pinholes as the film thickness shrinks, making the bubbles create a void between the substrate and the air interface.

So, what could be the possible solution for these problems? Let's find out by understanding about the role of defoamers in detail...
Role of Defoamers
Role of Defoamers
In addition to reducing surface defects on coated substrates, effective foam control agents are beneficial in preventing or reducing many common problems such as:
- Viscosity increase and loss of mechanic shearing power during milling (resulting in smaller batch sizes and poor dispersion of fillers and additives)
- Volume increase during mixing or shearing processes that leads to over-foaming
- Slower package-filler rates due to inefficient pumping
- Air incorporation during transport and handling, and
- Slower coating speeds or lower pressures during spraying1
Foaming is an emulsion of gas in liquid. All processes favoring gas formation and their introduction in liquids favor foaming as, for example:
- A low surface tension of the medium
- The generation of gas in-situ (temperature increase, pressure decrease, etc.)
- The incorporation of the surrounding gas, usually air into the liquid by mixing, pumping, spraying, etc.
Foam problems are especially prevalent during high speed processes that are used to formulate, mix, or apply the adhesive. These high speed processes are dynamic and new air-liquid interfaces are created very rapidly.
It is important in such applications for adhesive formulators to:
Choose a surfactant system or add a foam control agent that will provide the desired adhesive characteristics, yet not cause excessive foaming.
Selecting the proper defoamer/anti-foaming agent for a unique application can be a daunting task. This is because, the optimal anti-foaming properties will depend heavily on the distinct adhesive formulation and on the processes employed in formulation or conversion.
Different descriptors are used to describe the products designed to control or prevent foaming. The common ones are defined below. Most foam control products can serve either role and are used interchangeably.
- Defoamers are generally added to pre-existing foam to eliminate the foam
- Anti-foaming agents are added to prevent a formulation from foaming in the first place, and
- Air release agents remove micro-air bubbles from a liquid and help them to rise to the surface
After understanding the various solutions to encounter defoaming, let's first discover how foam is generated and the various mechanisms to control foam generation...
How Foam is Generated?
How Foam is Generated?
Pure liquids do not foam. If gas is incorporated into a pure liquid, it tends to form spherical bubbles since this involves the least amount of surface energy. However, if the liquid is pure, the bubbles will rise toward the surface and collapse immediately. As the air in the bubble is expelled, liquid quickly rushes into the space vacated by the air.
Thus, stable foam is never achieved in pure liquids
On the other hand, foaming will result if the liquid is not pure or if it is contaminated. Common contaminants can include:
- Minerals
- Salts
- Starches, and
- Metabolized wastes from microbes
In water-borne adhesives, these “contaminants” are generally additives used to impart specific properties such as reduced surface tension to the adhesive formulation. In water-borne adhesives these include:
Other additives can contribute to foaming as well. Table below describes how several common additives affect foam generation.
| Additive |
Characteristics |
|
Surfactants |
- Latex binder resins require surfactants in their production
- These tend to be anionic surfactants such as sodium lauryl sulfate
- The amount of surfactant used depends on the polymer being produced and the particle size desired
- In general, hard
- High Tg, small particle size lattices tend to foam substantially more than soft, low Tg, large particle size lattices
|
|
Dispersants |
- Dispersants generate foam in a process similar to surfactants
- They contain both a hydrophilic and hydrophobic portion
- The amount of foam generated depends on the relative size of these portions
- The greater the charge separation, the more the behavior resembles that of a conventional surfactant
|
| Coalescents |
- Coalescents also contribute to foaming
- Varying the coalescent can alter the viscosity and rheological response of a coating system.
- These also feature hydrophobic/hydrophilic structures
|
| Thickeners |
- Thickeners affect the rheological properties of any given adhesive
- Their increase in viscosity often causes an increase in entrained air
|
Effect of Common Additives in Water-borne Adhesive on Foaming Tendency2
Sometimes the surfactants that contribute to foaming are not deliberately added to the liquid but they either come from:
- Other formulation ingredients
- Accidental contamination in processing equipment
Gas can also be generated within an adhesive as a result of curing processes. This is especially evident in certain polyurethane adhesive systems. Also, application of the adhesive onto a porous surface may result in some of the entrapped air entering the liquid adhesive.
Surfactant Activity with Liquid System
The surfactants that are present will attach themselves to the gas bubbles that are entrained in a liquid adhesive and form an oriented interfacial layer around the bubbles. This is shown in the figure below:
Surfactant Activity within Liquid System3
As the bubble rises to the surface, a liquid lamella forms. The lamella is a thin layer of liquid that is actually trapped between two opposing surfactant treated surfaces, as shown in the figure below:
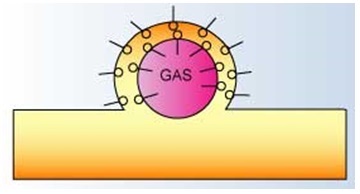 Surfactant Activity at Liquid - Air Interface3
Surfactant Activity at Liquid - Air Interface3
It can be represented as a double surfactant layer lined balloon. The hydrophilic end of the surfactant extends into the liquid lamella while the hydrophobic end resides at the surface of the liquid.
The interaction between the charges associated with the hydrophilic heads,
the atmospheric pressure and the gas pressure within the bubble result in a stable foam
As more and more of this lamella structures form at the surface interface layer, they begin to pack together. Also, they change from their natural spherical shape into polyhedrons, as the buoyancy force of the bubbles actually squeezes the liquid out from between the surfactant layers of the lamella.
In our exclusive guide, get selection & formulation tips for surfactants you can benefit from and the trends driving developments of new surfactants.

Foam Control Mechanisms
Foam Control Mechanisms
Foam control agents can be added to destabilize the foam or prevent any tendency for foam to occur.
The factors that have been found necessary for efficient foam control characteristics are indicated below:
- The liquid phase of a defoamer must have a degree of incompatibility or insolubility with the media into which it is placed.
- The liquid must have a rapid spreading coefficient so that it spreads across the media. This is generally evident in additives that provide a low surface tension.
- The foam control agents that are hydrophobic particles work on a semi-specific contact angle. So, they must have the correct size and shape. This enables the agent to penetrate the foam wall.
Foam control agents function by being more surface active than the surfactant that is stabilizing the foam. In this way, they are able to enter the surface layers of the potentially foaming liquid and displace it from the gas / liquid interface. The mixed surfactant layers will prevent close association of molecules in the original liquid. The thermodynamic factors and surface properties influencing the foam control mechanism and foam stability have been well covered in the literature.3,4
When a defoamer is added to a foamed system, it quickly spreads to a monolayer across the surface. This rapid spreading pulls the underlying liquid in the direction of the spreading defoamer. The outer lamella walls then begin to thin until they finally breakdown. The defoamer then spills through the hole into the liquid lamella and repeats the action on the inner lamella wall.
The efficiency of anti-foaming agents depends on the ability to spread them throughout the liquid and the ability to penetrate foam. The following parameters can be described as follows:
Penetration coefficient: E = σl – σd + σint
Spreading coefficient: S = σl – σd - σint
In which: σl = surface tension of original liquid
σd = surface tension of anti-foaming agent
σint = interfacial tension between defoamer and the liquid to be defoamed
- A defoamer can penetrate a foam containing liquid if E < 0, and
- A defoamer can also spread itself spontaneously within the liquid if S > 0
The efficiency of foam control agents is increased if
surface tensions and/or interfacial tensions are decreased
Anti-foaming agents therefore contain surface-active components. A low viscosity of the anti-foaming agent will also contribute to efficient penetration and spreading.
Composition of Foam Control Agents
Composition of Foam Control Agents
The composition of foam control agents is extremely diverse. They can be supplied as solids, pastes, or liquids. Liquids are the most predominant form. Foam control agents are typically composed of:
- A hydrophobic material:
Hydrophobic components are considered the most active ingredients in a foam control agent for reasons described above. Typical hydrophobic materials are:
- Treated silica
- Synthetic or natural waxes
- Polyglycol, and
- Silicones or silicone derivatives
These hydrophobes may either be used alone or in combination.
- A carrier vehicle:
Carrier vehicles are generally:
- Mineral
- Vegetable and silicone oils
- Alcohols
- Glycols, and water
The long-term stability of the carrier in the original liquid is important in achieving foam control with long shelf life products. The purpose of the carrier fluid is to transfer the hydrophobic active agents with some homogeneity into the hydrophilic system that is holding the air. The carrier fluid usually has a lower surface tension than the foaming liquid.
- Optionally, an emulsifier:
Emulsifiers ensure the optimum distribution of the hydrophobic component in the carrier, and it eases the spreading of the foam control agent throughout the liquid. The type and quantity of emulsifier to be used depends on the application (high shear forces, etc.). The minimum possible quantity of emulsifier should be used since emulsifiers themselves can contribute to foam formation. Emulsifiers typically used include:
- Ethoxylated alkylphenols
- Sorbitan esters
- Polyethylene glycol esters, and
- Others
The final foam control agent is a sophisticated blend of these materials along with secondary ingredients such as silica powder. For example, a ready-to-use foam control agent based on silicone can be formulated as follows:
- Polydimethylsiloxane (PDMS)
- Polyether modified PDMS
- Silica powder
- Emulsifiers
- Water or other aqueous carrier5
Types of Foam Control Agents
Types of Foam Control Agents
Several types of materials are available to achieve suitable anti-foaming properties as indicated in the figure below:
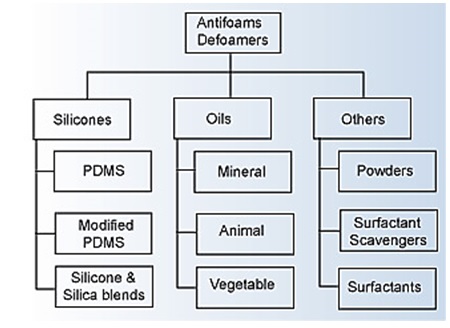 Major Families of Foam Control Additives5
Major Families of Foam Control Additives5
Basic foam control agents are generally classified as being silicone-based or non-silicone. However, there are a large number of commercial products that are blends of different components, and a large number have unspecified or proprietary chemistry.
Silicone-based Foam Control Agents
Silicone foam control agents are very popular in water-borne adhesive formulations because of their significant hydrophobic nature and water incompatibility. They also have a very low surface tension (20 mN/m). This makes them highly surface active. The simplest silicone polymer is polydimethylsiloxane (PDMS), an oil.
(CH3)3SiO-(Si(CH3)2O)n-Si(CH3)3
where n = 0 to > 1000
By appropriate modification of the silicone content in the molecule, the inherent hydrophobic nature can be changed.
Good results can be achieved with a relatively low Si-containing organo-modified siloxane
The main disadvantage of PDMS is that it is so insoluble that it is very difficult to disperse in water-borne systems and almost inevitably causes surface defects. To correct this problem PDMS is modified in the form of silicone-polyether copolymers. The copolymers are synthesized from reactive siloxanes and polyethylene / polypropylene glycol ethers.1,6 A range of modified PDMS structures is available.
Mineral Oils-based Foam Control Agents
Another large classification of foam control agents is based on mineral oils. These are insoluble in water, exist in a broad range of molecular weights, and present a large choice of chemical structure. As with silicone-based additives, mineral oils can be chemically modified to adjust their properties. Pure water and silicones are at two ends of a broad range of surface tension and solubility parameters.
Silicone-free Foam Control Agents
It is possible to formulate a completely silicone-free foam control agent, only based on organic substances, with an activity as good as a silicone based product. Popular non-silicone additives that can be used as non-foaming surfactants are acetylenic diol-based surfactants (e.g., Surfynol from Air Products and Chemicals).
These offer low molecular size and branching geometry that provides the low dynamic surface tension required for good wetting and also for a foam control product.5 They are claimed not to interfere with peel, tack, shear, and other properties of the adhesive. Also, they are available in an assortment of grades to meet a broad range of requirements for direct and indirect food contact.
Oils and other non-silicone foam control agents are aligned in intermediate locations as illustrated in figure below:
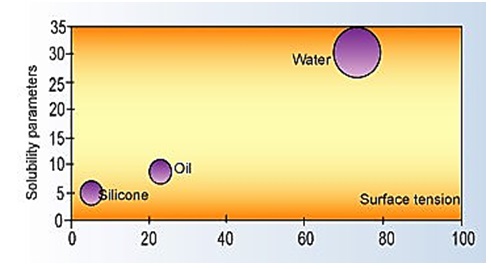 Solubility Parameters versus Surface Tension7
Solubility Parameters versus Surface Tension7
Role of Fillers in Defoaming
Filling with particles also influences defoaming. Inorganic fillers are well known silicas, fumed or precipitated. However, finely divided organic substances such as waxes can also be used. Silicone-containing agents are usually boosted by filling (organic or inorganic). With other organic foam control agents filling with inorganics does not improve the defoaming properties, but organic fillers will lead to an improvement. This gives two possible pathways for foam control agents:
- Based on silicone, and
- Without any silicone
In our exclusive guide, get an in-depth knowledge about fillers and extenders.

Selection of Foam Control Agents
Selection of Foam Control Agents
While formulating adhesives, it is critical to choose a foam control compound that provides the required anti-foaming properties without adversely affecting the adhesive characteristics. The
table below lists some critical properties of the adhesive formulation that can be affected by a foam control additive.
| Application and Coating Properties |
Performance Properties |
- Wetting (dynamic and static)
- Flow and leveling
- Mechanical stability
- Film integrity
- Surface smoothness
|
- Tack, shear, and peel
- Humidity resistance
- Clarity, color
- Adhesion of other materials to cured adhesive coating
|
Coating and Performance Properties of Adhesives Systems
that can be Affected by an Anti-foaming Agent
The choice of the foam control agent and its concentration level is a delicate balance between the technical requirements inherent to the product and process parameters that will be employed and economic constraints. This is shown in the figure below:
Defoamer Selection Balance1
Selecting the proper type of defoamer from the huge number of available products requires some basic selection criteria. Figure below illustrates an example of a decision tree that can be used in the selection process. Answers to specific process parameters, economics, and technical requirements are required to identify the right solution.
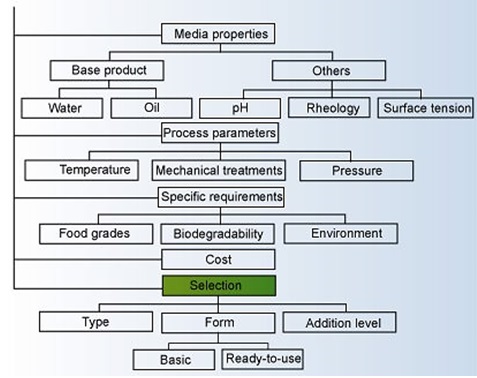 Decision Tree to Find the Right Foam Control Agent2
Decision Tree to Find the Right Foam Control Agent2
The applicability of a defoamer strongly depends upon the specific adhesive system and processes utilized in formulating and applying the adhesive. One of the main selection criteria to be made is the choice between silicone-based or silicone-free defoamers. Table below provides a breakdown of the various foam control agents.
| Base Polymer
|
Silicone-Based
|
Silicone-Free
|
|
Micro
crystalline Waxes
|
Mineral Oils
|
Polymerics
|
Silica-Based
|
Surfactants and
Fatty Acids
|
Unspecified
|
|
Acrylics and acrylic copolymers
|
✓
|
✓
|
✓
|
✓
|
✓
|
✓
|
✓
|
|
Aminoplasts / phenoplasts
|
|
|
|
|
|
✓
|
✓
|
| Epoxies
|
✓ |
|
|
|
|
|
✓ |
|
Ethylene co-terpolymers - emulsions
|
|
|
|
✓
|
|
|
|
|
Ethylene co-terpolymers – solids
|
|
✓
|
|
|
✓
|
✓
|
✓
|
|
Natural rubbers
|
|
✓
|
|
|
|
|
|
|
Natural based polymers (animal, vegetable)
|
|
|
|
|
|
|
✓
|
|
Polyamides
|
|
|
|
|
|
|
✓
|
|
Polychlorovinyls
|
|
✓
|
|
|
|
|
✓
|
|
Poyesters
|
✓
|
|
|
|
|
|
✓
|
|
Polyolefins
|
|
|
|
✓
|
|
|
✓
|
|
Polysulfides
|
|
|
|
✓
|
|
|
✓
|
|
Polyurethanes |
✓
|
|
|
|
|
|
✓
|
|
Polyvinyl acetate emulsions and derivatives
|
✓
|
|
✓
|
✓
|
✓
|
|
✓
|
|
Polyvinyl alcohols
|
✓
|
|
✓
|
✓
|
✓
|
✓
|
✓
|
|
Polyvinyl pyrrolidones
|
|
|
|
|
|
|
✓
|
|
Silicones
|
✓
|
|
|
|
|
|
|
|
Silyl-modified polymers
|
|
|
|
|
|
|
✓
|
|
Styrene block copolymers
|
|
|
|
|
|
|
✓
|
|
Styrene copolymers
|
|
|
✓
|
✓
|
✓
|
|
|
|
Synthetic rubbers
|
✓
|
|
|
|
✓
|
✓
|
✓
|
Defoamers / Anti-foaming Agents for Specific Adhesive Chemistries
Silicone- or polysiloxane-based defoamers excel in low surface tension and good solubility. This makes these products first candidates for use in solvent borne systems, where the low surface tension of the solvent has to be passed in order to create good defoamer spreading features.
Furthermore, polysiloxanes are preferred in clear systems for reasons of not effecting transparency. However, polysiloxanes are more sensitive for effecting adhesion properties and are typically more expensive compared to polysiloxane-free defoamers.
Optimal defoaming properties are obtained by ensuring limited compatibility of the defoamer with the foaming system. Poor compatibility results in high risk for creating film surface defects, whereas excellent compatibility generally means poorer defoaming properties.
Tests to Determine the Efficiency of Defoamers
Tests to Determine the Efficiency of Defoamers
Several test methods are known for determining the efficiency of defoamers. It is important that one considers both the type of formulation being modified as well as the practical processing (formulating and application) conditions that will be used in practice. Testing in liquid non-aqueous systems is often difficult, as the amount of foam is typically much less compared to aqueous systems.
It is essential to select a test method that is most closely related to the actual application or foam generation mechanism. This starts with analyzing the highest risk of foam formation. For example, roller application, dipping, spraying, etc. are conditions of intensive contact between the liquid formulation and air and provide the greatest risk of foam formation. Depending on main risks in practice, the best corresponding laboratory test method is chosen.
Agitation Test
A method related to dispersion or mixing conditions is the agitation test. This can be accomplished using either a paint shaker or a high speed mixer with a dissolver disc.
The common paint shaker is often used to simulate foaming in formulations. The various defoaming candidates should be evaluated at two or three concentrations. And the modified formulations should be shaken for several minutes. After this, the degree of foaming may be visualized or if qualitative results are required, the specific gravity of the formulation is determined - the higher the specific gravity, the lower the presence of foaming.
An alternative method is to stir about 50 gms of modified formulation (about one minute for low to medium viscosity formulations and three minutes for high viscosity formulations) at high speed (3000 rpm) with a dissolver disc. This incorporates and finely disperses a large amount of air into the formulation. Immediately after stirring, the formation is poured onto a transparent polyester film fixed to a glass panel inclined at a 25° angle. After drying, the coating film can be assessed visually.
Application Test
Another type of test is the assessment of a coated adhesive film after application. The presence of foam or microfoam is judged using a microscope or magnifier. Gloss, haze, and color of the applied film may also be an indication of foam formation.
Shelf-life Test
It is essential to do repeat testing after storage of the modified formulation. As stated earlier, defoamers are chosen to have limited compatibility. Consequently some risk of separation or adsorption may occur during storage. Typically the modified formulation is aged for several weeks at a slightly elevated temperature (about 50°C) and then retested for defoaming efficiency.
Find Suitable Defoamer & Anti-foaming Agent Grade
View a wide range of defoaming & anti-foaming agent grades available in the market today, analyze technical data of each product, get technical assistance or request samples.
References
- O’Neil, V., et. al., “New Silicone Foam Control for Waterborne Coatings”, Paint and Coatings Industry, October 2003, pp. 54-62.
- Cappablanca, C.J., “Foam Control Agents for Aqueous Architectural Paint and Industrial Coatings”, Paint and Coatings Industry, February 1997.
- Garrett, P.R., “Defoaming: Theory and Industrial Applications”, P.R. Garrett, ed., vol. 45, Surfactant Science Series, Marcel Dekker, New York, 1993.
- Porter, M.R., Handbook of Surfactants, Blackie and Son Ltd. Chapman Hall, New York, 1991.
- “Foam Control Additives: Defoamers, Antifoaming Agents, Antibubble Additives, Foam Suppressants”, SpecialChem4Polymers.com, December 8, 2004.
- O’Neil, V., et. al., “Raking in the Benefits of Silicone Defoamers”, Asia Pacific Coatings Journal, June, 2004, pp. 22-24.
- Briones, J. and Shah, V., “Surfactants Offer Benefits to Adhesive Formulators”, Adhesives and Sealants Industry, February 2004.