Need for Dispersing Agents
Need for Dispersing Agents
A dispersing agent is necessary to:
- Generate a stable formulation, and
- Provide storage stability
Thereby eliminating viscosity change and phase separation.
The dispersants deflocculate solids and thereby significantly
reduce adhesive viscosity. Alternatively, solid loading can be increased with minimal effect on the formulation’s viscosity.
Dispersing agents are used in:
- Both water-borne and solvent-borne adhesives and sealants of all types.
-
Water-borne formulations and are often combined with wetting agents to achieve the required balance of properties for the particular application.
Their selection depends primarily on the other ingredients in the formulation and the final properties that are necessary for the specific application.
Let's explore the mode of action
of dispersing agents and their stabilization mechanism in detail...
Dispersing Agents – Mode of Action
Dispersing Agents – Mode of Action
In order to provide optimal performance, filler particles must act independently of each other. Thus they must remain well dispersed throughout:
- Manufacture
- Storage
- Application, and
- Adhesive film formation
Unfortunately, most dispersion are inherently unstable, and they must be stabilized against the flocculation that inevitably occurs in unstabilized systems. To this end, dispersing agents, usually together with wetting agents, are added to the adhesive formulation.
Manufacturers often employ the terms “wetting agent”, “dispersing agent”, and “wetting
& dispersing agent” indiscriminately because the distinction between them is not very clear.
-
Wetting agents (surfactants) are low molecular amphiphilic substances (molecules having a polar water-soluble group attached to a water-insoluble hydrocarbon chain). An effective wetting agent for coating should be a surfactant that has affinity groups to solid particles. Also, it must be able to replace air and moisture that traps in the solid particles in order to spread and penetrate to the surface of the particles.
- Dispersants are also relatively low molecular weight with a large number of pigment affinic / charge donating groups. These are only used in aqueous formulations. They do not reduce the interfacial tension of the particle-binder interface. Therefore, they must be used in combination with wetting agents to be suitable.
- Wetting and dispersing agents can be lower or higher molecular weight polymeric organic molecules with long polymeric tails and one or more pigment anchoring groups. They are used in both aqueous and non-aqueous systems to deflocculate and stabilize particles in the formulation. These additives perform the function of both wetting agents and dispersing agents noted above.
Dispersant and wetting agent combinations are very common. They are able to enhance dispersion quality, and reduce dispersion time significantly.
Here, “dispersants” and “wetting and dispersant agents” as defined above will be referred
to collectively as dispersing agents.
In terms of chemical structure one can usually divide dispersing agents into the two following classes:
- High molecular weight polymeric dispersants
- Low molecular weight dispersants (surfactants)
The main distinctions between those two types of dispersants are:
These differences are summarized in table below:
| Differences |
Low Molecular Weight
Dispersing Agents |
Polymeric
Dispersing Agents
|
| General effects |
- Reduce surface tension to facilitate wetting
- Use differences in charge to perform the anchoring process
- Use repulsion of same charges and attraction of different charges for stabilization
|
- Reduce surface tension to facilitate wetting
- Does not use differences in charges to perform anchoring process
- Use steric hindrance for stabilization
|
| Chemistry |
- Low molecular weight surfactant
- Most of the time containing ionic groups for stabilizing mechanism
|
- Functional copolymers with special chemical groups for stabilizing mechanism
|
| Molecular weight |
500 - 2000 |
4000 - 25000 |
| Dosage (solids on particle weight) |
0.4% - 5.0% |
2% - 40% |
Main Differences between Low Molecular Weight and Polymeric Dispersing Agents
As the adhesive binder or carrier penetrates into the pigment agglomerates and wets the surface of the primary particles, the wetting stage is continuously transformed into a pigment dispersing stage. The figure below shows the same:
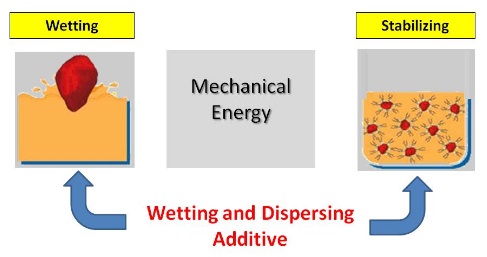 The Wetting and Dispersing Process
The Wetting and Dispersing Process
The pigment dispersing stage may require the input of energy. Usually it requires mechanical energy, to break down agglomerates. Air and moisture entrapped at the particle surface are displaced to the medium’s liquid phase, and the pigment / air interfaces become pigment / liquid interface. To proceed, the liquid needs to wet the particle surface.
Dispersing agents are usually fairly low molecular weight materials. They strongly adsorb onto particles and form a repulsive barrier to the positive forces of interaction. These barriers exist between all particulate materials. In many solvent-based
systems a portion of the film-forming polymer may provide this function. But in aqueous adhesives (especially latex systems) separate dispersants are usually added.
Stabilization Mechanisms
Stabilization Mechanisms
Wetting and dispersing agents prevent particles from flocculating by:
The combination of both these stabilization mechanisms is called electrosteric stabilization. The importance of each stabilization mechanism may change during film formation. This is because, the polarity of the film may continuously change due to:
- Evaporation of solvents and water, and
- Any chemical reactions of the binder ingredients
The two processes are shown in the figure below:
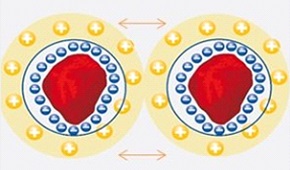
| Electrostatic Stabilization |
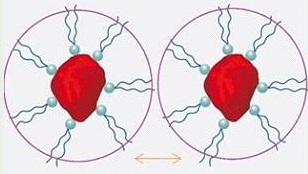
Steric Stabilization |
 |
 |
- Pigment particles have the same surface charge
- Charges are arranged in double layer causing the repulsion
- Vander-waals forces cause the attraction
- Mainly for inorganic pigments and dispersions in water
- Stability can be affected by high salt concentrations
|
- Liquid phase soluble polymer chains absorbed to the pigment particles through the anchoring groups
- Strong stabilizing mechanism
- OK for water-borne and solvent-borne systems
|
Electrostatic Stabilization
Electrostatic Stabilization
Inorganic fillers dispersed in water mostly bear electric charges. Since the overall dispersal system is uncharged, the liquid phase must contain an equal number of counter ions in close proximity to the particles. An electric double layer (ion cloud) forms and flocculation is prevented. This is because, of the electrostatic repulsion of like charges. There are various mechanisms by which the surfaces of filler particles can acquire charges:
- Dissociation of functional groups on the particle surfaces
- Adsorption of ions (mostly polyanions)
Electrostatic stabilization is especially important in water-borne systems. This is because of the high dielectric constant of water. However, studies have shown that the electric charge on particle surfaces also plays an important role in solvent borne paints. The same particle dispersed in different resins may exhibit opposite charges or none at all.
Additives classified as “wetting agents” provide some electrostatic repulsion but not very strongly. “Dispersants” and “wetting and dispersing agents” provide a high degree of electrostatic pigment stabilization.
Steric Stabilization
Steric Stabilization
Aqueous dispersions can be readily stabilized against flocculation. This is could be done by adding water-soluble polymers (so-called protective colloids), such as:
In contrast to electrostatic stabilization, stabilization effected with polymers is insensitive to the addition of electrolytes (salts).
Wetting agents (surfactants) can also act as steric stabilizers. Low molecular weight wetting agents do not act as steric stabilizers as their molecules are much too small. Only oligomers or polymers have the necessary molecular size.
The requirements for steric stabilization are:
- The polymers must strongly adsorb on the surface by appropriate functional groups (anchoring groups).
- The polymers must have sufficiently long chain segments (barrier groups). They should readily dissolve in the dispersion medium (organic solvents or water), a process that leads to widening of polymer chains. The addition of poor solvents can cause these polymer chains to coil and so lead to flocculation.
- Polymers (oligomers) of medium molar mass are optimal. If the molar mass is too low, the chain is not long enough. If the molar mass is too high, bridging flocculation may occur. Moreover, if the molar mass is too high, incompatibility may occur or the viscosity may increase.
- A minimum polymer concentration is necessary. If the concentration is too low, flocculation may occur, especially in the case of high molar masses.
Chemical Classification of Dispersing Agents
Chemical Classification of Dispersing Agents
Dispersing agent additives are produced using many different types of chemicals depending on the application in different industries. They can be lower or higher molecular weight organic molecules having the following characteristics:
- Long polymeric tails for steric hindrance –
The polymeric tails, responsible for steric hindrance, can be simple long alkyl chains (e.g., from fatty acids) or more complex polymers (e.g., polyether, polyacrylates, polyurethanes, polyesters, or their combinations).
- One or more anchoring groups to adsorb onto the particle’s surface and donate charge –
The most common anchoring groups are:
- Carboxylic
- Tertiary nitrogen
- Quaternary ammonium
- Nitro
- Phosphate, and
- Borate
However, practical considerations limit the selection because of negative side effects such as:
- Water resistance
- Reduced corrosion protection, and
- Others
The carboxylic and tertiary nitrogen anchoring groups are universal and suitable for many different types of particles.
Wetting and dispersing additives are traditionally classified as being:
- Cationic
- Anionic, or
- Electroneutral
Non-ionic products are widely used in aqueous systems. Many newer additive molecules actually contain cationic, anionic, as well as non-ionic segments.
As discussed earlier, the two major chemical groups of wetting and dispersing
chemistries are comprised of -
- Lower molecular weight polymers: Various derivatives of fatty acids or vegetable oils
- Higher molecular weight polymeric dispersants: Block polymers, containing blocks of anchoring groups and polymer groups for compatibility and steric hindrance
The fundamental chemical structure of most high molecular weight dispersants are:
The reason these three basic groups are chosen is due to their wide compatibility with most common resins.
Classification of Polymeric Dispersing Agents
The classification of polymeric dispersing agents is based on their anchoring mechanism and their molecular weight. They are also influenced by the polymer design (linear, branched, star designed) and the polymerization process. Specifically designed, polymeric wetting and dispersing agent offer very high quality:
- Many anchoring groups
- Large choice of chemistry and large choice of polymer design and molecular weight
- Mw = 5000 ~ 50000 g/mol
- Excellent wetting power and dispersing time reduction
- Very effective for the long term stabilization
- Suitable for water-borne or solvent borne formulations and organic or inorganic particulates)
Some special types of polymeric dispersing agents with branched structures are not only able to deflocculate pigments but are also capable of building some secondary structures. The structures that are developed are due to the associations between the wetted pigment particles. It provides a weak network of pigments and extenders, thereby preventing pigment settling and improving sag resistance.
Combinations of lower molecular weight wetting agents and higher molecular weight polymeric dispersing additives can produce well established dispersion. That too, at much lower viscosities than either additive used alone.
The table below summarizes the value propositions that are provided by specific wetting and dispersing agent chemistries.
| Wetting and Dispersing Agent Types |
Characterization and Value Propositions |
| Conventional surfactant types |
- They have an excellent compatibility and excellent water dispensability
- Offer many alternatives to replace the APEO (alkylphenol ethoxylated) products
- Reducing the surface tension improves the wetting process
|
| Gemini surfactants |
- Ionic and non-ionic surfactants
- They have at least two hydrophobic and two hydrophilic groups in the molecule
- Gemini surfactants are characterized by unusually high interfacial activity
- In addition to a very good substrate wetting, some products show very low foaming tendency
|
|
Fluorosurfactants |
- The hydrophobic part of the chain has all the hydrogen atoms attached to carbon replaced by fluorine atoms
- They are characterized by high polarity, high thermal and chemical stability, and high resistance to UV radiation
- They repel dirt, oil, fat, and water
|
| Modified fatty acid types |
- Can be used to stabilize both organic and inorganic particles
- They provide excellent results for universal pigment concentrates
- Their water resistance is limited
|
| Phosphoric acid ester |
- Polyether and polyester structure
- Excellent for inorganic material dispersion
|
| Alkyl phenol ethoxylate (APE) |
- Have very broad dispersing properties and low cost
- Suitable for both water-borne and solvent based systems
- The use of APEs is becoming restricted because of environmental regulations
- They are being replaced by modified fatty acid ethoxylates and modified polyethers
|
|
Polyacrylic acid based
|
- Usually lower in molecular weight (and also in cost) in comparison with the other structures
- They are particularly recommended in water-borne systems to increase the pigment load of inorganic material
- Ammonium and sodium salt are typical products for latex paints
|
| Polyurethanes |
- Excellent for viscosity reduction
- Enhance the solids content and reduce the dispersing time
- The flexibility of this structure (backbone, branched chains, anchoring groups) allows the design of various structures for many solvent borne and solvent free systems
|
|
Polyacrylates
|
- They have similar properties to the polyurethanes
- Higher in molecular weights, they can offer better compatibility where the polyurethane structure is not acceptable
- Suitable structure for water-borne and solvent borne systems
- Can be modified to provide excellent water resistance
|
| Maleic anhydride copolymers |
- Does not allow the broad variety of structures as do the acrylates
- But enables development of dispersing agents for all kinds of pigments
- These additives are very resistant to water
|
| Controlled polymerization technology / living chain growth |
- Allows the manufacturer to make very fine adjustments on the polymer chain, which is not the case with the classical step-growth process
- Additives are very similar batch to batch. These are very effective but more expensive products
|
Value of Wetting and Dispersing Agents by
their Chemistry
Formulation Challenges
Criteria for Selecting Dispersing Agents
Criteria for Selecting Dispersing Agents
Dispersant agent selection involves a number of criteria, such as:
-
Carrier
-
Base resin involved, and
-
Surface nature of the particle
All of them can affect the performance of the additive. Dosage requirements are mainly dependent on the type of particle to be dispersed and its surface area.
Let's understand these criteria in detail...
Carrier
1. Solvent-borne Systems
In solvent-borne systems, the main stabilization mechanism is steric hindrance. But the electrostatic charge repulsion can also play a role, especially in higher polarity solvent-borne
systems. The solvent blend used must be a good solvent for the additive itself.
» Explore the Dispersing Agents for Solvent-borne Systems!
2. Water-borne Systems
In aqueous inorganic pigments, stabilization is entirely by charge stabilization.
Higher the polarity of the system, greater is the effect of stabilization by electrostatic repulsion. The stabilization of pigments in aqueous systems can be compromised by the presence of cations.
Multivalent cations are especially disruptive as they tend to form insoluble salt particles that act as nuclei for initiating flocculation. Dispersion agents are able to neutralize and complex these cations, keeping them in soluble form, to enhance colloidal stability.
» Select the Dispersing Agents for Water-borne Systems!
3. Solventless Systems
Wetting is a relatively simple process in solvent-borne systems. Most binders have a surface tension of 25-30 dynes. Solvent-free binders usually have a higher surface tension and higher viscosities than solvent-borne resins. Therefore, their wetting properties are less pronounced compared to their solvent-borne counterparts.
Wetting in aqueous systems is more difficult, because water has a surface tension of 72 dynes and most aqueous binders also exhibit a higher surface tension than comparable solvent-borne binders.
Base Resin
Technological progression has resulted in the creation of more and more sophisticated resins, such as:
These resins may have very bad wetting properties to the particle surface. Thus, wetting and dispersing agents are necessary.
Each additive used for pigment stabilization must be compatible with the binder. Incompatibility often results in phase separation and poor dispersions. Slight incompatibility problems can often be corrected with the proper choice of solvent or solvent blends.
Surface Nature of Particle
The choice of dispersant is also related to the surface nature of the particle. The polarity of the surface differs from organic (non-polar) to inorganic (more polar). This means that the nature of the dispersant anchor group is critical for optimum adsorption.
The choice of anionic anchor group should allow for better performance with inorganic particles. And a cationic anchor group should be more appropriate for organic particles.
The particle surface area affects the level of dispersant required. If too little is used then the full benefits will not be realized. If too much is used, it can be shown that the thickness of the protective barrier is actually reduced as a result of overcrowding on the pigment surface. Therefore, use of an excess level of dispersant leads to final coating properties. These are inferior to those obtained with an optimized dosage.
Film properties such as adhesion or hardness can also be adversely affected by the use of an excess of dispersant because of the free molecules in the drying film.
An efficient and stabilized dispersion can only be reached if almost all particle surfaces are wetted and covered by the dispersant. Therefore, the dosage of dispersant is strongly dependent on the surface area of the pigments. High molecular weight dispersants need higher amount of dosage than low molecular weight dispersing agents.
An estimate of the dispersing additive demand can be made if the surface area of the pigments is taken into account. The table below indicates the recommended dosages of high molecular weight dispersants:
| Particle Type |
Concentration Based on Particle Weight |
| Titanium dioxide |
2-3% |
| Iron oxide |
3-4% |
|
Fillers (clay,
calcium carbonate, etc.) |
1-2% |
| Regular carbon black |
20% |
| High channel carbon black |
30-40% |
Recommended Dosage of High Molecular Weight Dispersant on Several Common Particle Types
Requirements for Proper Functioning of Dispersing Agents
Requirements for Proper Functioning of Dispersing Agents
In order for a dispersing agent to function properly, it must have the flowing requirements:
1. Compatibility
The simplest way to determine compatibility is to mix the dispersing agent into the formulation without particulate fillers. It should be perfectly compatible with the other formulation components and not show any:
- Color change
- Phase separation
- Viscosity increase, or
- Other changes in important properties
One can also check compatibility by doing a draw-down and observe the color strength and transparency of the film.
- For inorganic pigments: It should be opaque
- For organic pigments and carbon black: It should have high transparency
These methods do not indicate the long term stability of the system.
2. Long-Term Stability
Long-term stability is the most important factor. This is because, it will reflect the performance ability of the dispersants. A fast check for long term stability would be to store the fully formulated product at elevated temperatures (50°-60°C) for two weeks. And then check viscosity and color. Insufficient storage stability might lead to flocculation.
3. Viscosity Determination
Dispersing agent additives can have very different effectiveness which leads to different dispersing additive demands. Usually, it is desirable to identify the minimum of the viscosity curve as shown in the figure below:
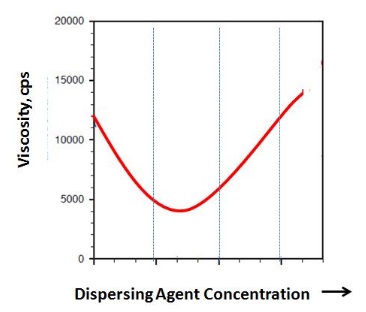 Dispersant Demand Curve of a Dispersing Agent and
Resulting Viscosity
Dispersant Demand Curve of a Dispersing Agent and
Resulting Viscosity
Since the shape of the curve might vary from dispersing additive to dispersing additive, it is important to not just replace an additive 1:1 as this might lead to incorrect results. At least three points along the viscosity curve should be measured to determine the shape of the curve. To add some safety margin, it is recommended to add at least 10% extra dispersing agent to the determined additive amount.
Find Suitable Dispersing Agent Grade
View a wide range of dispersing agent grades available in the market today, analyze technical data of each product, get technical assistance or request samples.