Importance of Rheology in Formulations
Importance of Rheology in Formulations
Rheology is the study of flow and deformation of matter. For many applications the formulation’s optimum viscosity will depend on the state of the product i.e., during compounding, while setting on a shelf, during application, immediately after application, and once in a joint configuration.
Although most product data sheets will define the viscosity by a single viscosity measurement, optimal application properties often require the viscosity to be measured and controlled at several shear rates.
During its life cycle, an adhesive must conform to several processes having different shear rates. Each of these processes may have different rheological demands. Different shear rates that are associated with various life-cycle processes are illustrated in the figure below.
Various options exist to adjust the viscosity; however, rheology modifiers are used to control viscosity over specific shear rates that are important to both the formulator and end-user.
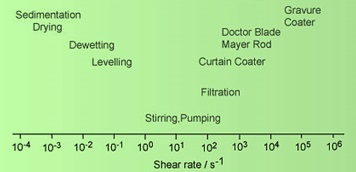 Characteristic Shear Rates During Application of PSA
Dispersions[1]
Characteristic Shear Rates During Application of PSA
Dispersions[1]
The table below shows how liquid rheology affects different processes. Rheology is especially important for:
- Adhesives that must be applied as coatings (e.g., pressure-sensitive
adhesives, laminating adhesives)
-
Adhesives that must not flow or sag before cure (e.g., adhesives that are applied to vertical surfaces, adhesives that must maintain their bond thickness during cure, and sealants that must resist sagging).
| Process |
Rheological Effect |
| Mixing and pumping |
Rheology affects the ease in which an adhesive is mixed during compounding or before application. Similarly, it affects the ease in which it can be pumped or transferred. Ease of mixing and pumping reduces process time and energy costs. |
| Settling and storage
|
The adhesive’s rheology is a major factor determining its storage or shelf life. Rheology will affect the degree to which settling occurs during storage and transport.
|
| Dispensing
|
Rheology determines how an adhesive can be applied (roll coating, extrusion, trowel, etc.). It also determines how the adhesive conforms to or “holds” a specific geometric pattern (lines, dots, vertical extrusion, etc.).
|
| Application
|
Flow control is important in determining the application process and how fast the process runs.This is especially important in the production of tapes, labels, and laminates. Rheology is also important in controlling wetting and penetration into porous substrates and sag resistance.
|
Effect of Adhesive Rheology on Various Processes
Difference Between Rheology Modifiers & Thickeners
Rheology modifiers and thickeners are used in water-borne & solvent-borne adhesives, and sealants of all types.
- They are used extensively in water-borne formulations, and often two or more are used to achieve the required balance of properties for the particular application.
- They are used to a lesser extent in solvent-borne formulations where they are primarily used to control viscosity and provide non-sag properties.
There is a difference between rheology modifiers and thickeners, although both terms are considered interchangeable in commerce. They are often considered together since their primary function is to modify the viscosity profile.
The following are differences between a rheology modifier and a thickener. However, for the purpose of this guide, they will be considered together since their selection is usually done as a single step in the formulation process and in certain applications they may be used together.
| Rheology modifiers |
Thickeners |
- Optimize the viscosity at a specific shear rate or range of shear rates
-
They can be organic or inorganic
-
Organic rheology modifiers do not usually increase the solids content of the formulation
-
More targeted type of thickener
|
- Increase the viscosity (resistance to flow) at relatively low concentrations and generally at low shear rates
- These are typically inorganic
- Inorganic materials that will increase the solids content and provide a degree of thixotropy
|
Before we dig into the use of rheology modifiers to control the flow behavior, it may be useful for you to understand the basic principles of rheology and different rheological processes in adhesives and sealants.

Let's explore the role of modifiers & the factors that a formulator should keep in mind to fulfil the manufacturing needs.
Role of Rheology Modifiers for Desired Flow Behavior
Role of Rheology Modifiers for Desired Flow Behavior
Rheology modifiers play a key role in achieving a desired viscosity profile, coating performance, non-sag properties, thickness build, adhesion, and other properties related to the flow (rheological) properties of the formulation.
There are a number of occasions when a formulator may want to consider using rheology modifiers. They depend on the internal manufacturing needs of the formulator and on the end-use application requirements. Listed below are several value propositions offered by rheology modifiers.
Possible Occasions When Rheology Modifiers May Provide an Added Value
- Reduce settling/increase shelf life
- Reduce time and energy cost for mixing and pumping
- Increase thickening efficiency and reduce raw material costs (use less product and keep the same performance level)
- Reduce splattering during adhesive manufacture and application
- Increase manufacturing or converting speed especially in tape, label, and laminate production processes
- Reduce penetration into porous substrates
- Increase sag resistance
- Increase coating build (thickness) per application
- Reduce open time or drying time
- Improve sprayability, brushability, other application processes
- Improve printability and resolution of adhesive coatings
|
Organic rheology modifiers generally do not affect the performance properties of the adhesive once it is applied and dried or cured. This is mainly because of the low concentrations (0.1 to 2.0 percent by weight) of rheology modifiers used in the water-borne formulation. However, certain properties such as moisture resistance of the final adhesive film can be affected by these modifiers, and testing is recommended.
Effects on Adhesion
Adhesion can also be affected by the addition of rheology modifiers, although studies in this area are limited. The relationship between good wetting and flow is apparent but not clearly understood. Of course, efficient mixing, long shelf life, and good coatability can also affect the quality and consistency of the resulting adhesive bond.
Inorganic thickeners can also provide improved joint characteristics by reinforcing the resin matrix or by modifying the adhesive’s coefficient of thermal expansion.
Cost Reduction with Rheology Modifiers
Viscosity modification can also offer a major opportunity to reduce costs and enhance performance at several instances. Some of them are listed below.
- Rheology modifiers can provide a gain in productivity and a reduction in energy consumption. New rheology modifiers are supplied as low viscosity liquids that are easy to mix. Once incorporated, the formulation is easy to pump via bulk handling and metering/mixing equipment.
- By using rheology modifiers converters can apply adhesive coatings at faster speeds without difficulty. This is especially important in high-speed tape, label and laminate manufacturing operations.
- Use of rheology modifiers permits formulations that exhibit less shear thinning, so that viscosity at high shear rates can be higher and, as a result, thicker wet films can usually be applied in one coating.
- Costs related to waste are also minimized since the formulations can be non-splattering, non-sagging, and subject to easy clean-up.
- Anti-settling properties will reduce costs associated with the degraded liquid product due to storage.
- Waste due to excessive penetration into porous substrates such as corrugated boxes and wood products is also minimized by controlling viscosity.
Meet specific rheological properties (optimal flow, faster drying...) in your WB adhesives with a smart selection of rheology modifiers as well as learn how can you achieve more with lesser ingredients by understanding the potential value of using the correct rheology modifier in this exclusive course by Adhesives Industry expert Ed Petrie.
Rheology Modifiers Chemistries - Which one suits you best?
Rheology Modifiers Chemistries - Which one suits you best?
Generally, rheology modifiers can be classified in two ways. First according to the suitability for the system - water-borne, solvent-based, or solvent-less formulations and another method is classification into inorganic and organic chemistries.
- In water-borne systems, rheology modifiers are used extensively to adjust the formulation’s flow properties for a specific range of shear rates.
- While, in solvent-based or solvent-less systems, rheology modifiers are added to increase viscosity and/or provide thixotropic (non-sag) properties.
Today, there are many materials that can be used as rheology modifiers, and each material has various grades and chemical variations that will affect rheological performance. All in all, there are hundreds if not thousands of rheology modifiers from which to choose. You can find different chemical variations and commercial grades of rheology modifiers available in our Adhesives Ingredients material selector database.
The figures below provide a classification scheme of the various inorganic and organic rheology modifier technologies that are commonly used with adhesives and sealants. These additives are also often used in coating formulations since their value proposition and mechanism of viscosity modification are similar.
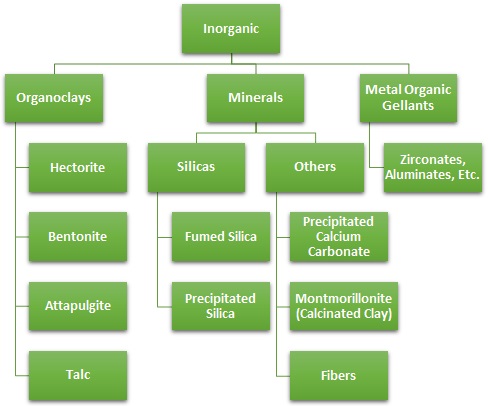 Common Inorganic Rheology Modifiers
Common Inorganic Rheology Modifiers
Common Organic Rheology Modifiers
The table below will familiarize you with various inorganic and organic rheology modifiers suitable for waterborne, solvent-based, or solvent-less formulations.
| System |
Typical Rheology Modifier |
| Organic |
Inorganic |
| Water-borne |
- Natural gums
- Cellulosics
- Alkali-soluble and alkali swellable emulsions (ASE)
- Hydrophobically modified alkali swellable emulsions (HASE)
- Hydrophobically modified ethoxylated urethanes (HEUR)
- Others
|
|
| Solvent-based |
- Castor oil derivatives
- Polyamides
- Calcium sulfonate derivatives
- Modified polyurea
- Others
|
|
| Solvent-less |
|
- Organoclays
- Minerals
- Fibers
|
Rheology Modifiers Typically Used in Various Types of Formulations
Organic Rheology Modifiers for Waterborne Systems
Organic Rheology Modifiers for Waterborne Systems
Let's begin by understanding the primary types of organic rheology modifiers that are used in water-borne systems. Overall, waterborne systems use two mechanisms to achieve viscosity modification - associative modification and non-associative modification.
Associative Modification Mechanism
Associative thickeners form intermolecular associations with dispersed particles (resin or filler).
- These interactions are the result of the presence of insoluble hydrophobic groups along the water-soluble thickener’s molecular backbone.
- Once added to water, the hydrophobic groups assemble into aggregates or micelles, and a three-dimensional network is formed which causes the medium’s viscosity to increase.
- The formation of hydrophobic domains are separated during shear conditions and become interwoven with the polymer chains.
- The domains will reform once the shear has been removed, at a recovery rate that is dependent on polymer structure, thickener concentration, and several other factors.
- The thickening effect will depend on the type and number of hydrophobic groups per polymer chain and the molecular weight of the modifiers.
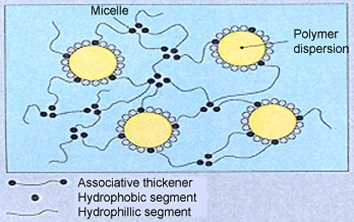 Mechanism of Associative Thickening[2]
Mechanism of Associative Thickening[2]
Factors that need to be considered when formulating with associative rheology modifiers include the presence of co-solvents and the order of component addition.
- Selected co-solvents (especially alcohols) can interfere with the associative domains and suppress the associative mechanism.
- The order of addition of formulation components will have an impact on processing and may affect the overall performance of the compound.
Associative rheology modifiers are considered to be highly efficient and versatile as well as attractive in higher viscosities at high shear rates (roll coating for example) lead to good film build per application with little spatter. They overall improve the stability of the system by coating the latex particles or filler particles with a protective layer that helps to prevent coagulation and settling during storage.
However, there are certain disadvantages linked with associative thickeners, which include hydrolytic instability, phase separation, and sensitivity to formulating changes.
Associative mechanism utilizes rheology modifying chemistries, such as HASEs and HEURs. Thus, they are also called associative modifiers.
#1. Hydrophobically Modified Alkali Swellable Emulsions (HASEs)
HASEs are produced by the addition of hydrophobic groups to ASE polymers. They provide greater control of compound rheology over a broader range of shear rates than the ASE class of thickeners. These associative modifiers are ionic substances that are dependent on alkali for activation of the thickening mechanism. HASEs are produced in emulsions at about 30% solids. They are designed for formulating coatings and adhesives that have a high film build.
Hydrophobically modified alkali-swellable emulsion (HASEs) rheology modifiers provide the formulation with good leveling properties along with anti-settling and sag resistant properties (when incorporated with pigments or extenders). They also provide formulations with good water resistance.
#2. Hydrophobically Modified Ethoxylated Urethanes (HEURs)
Hydrophobically modified ethoxylated urethanes (HEURs) typically consist of polyethylene glycol units of varying lengths, connected by urethane linkages and terminated with hydrophobic end groups. Unlike the ASE and HASE classes of thickeners, these rheology modifiers are non-ionic substances, and they are not dependent on alkali for activation of the thickening mechanism.
HEURs are typically supplied as a 20-50% solids emulsion. Certain HEUR products are available as low VOC and tin-free versions. They can be used alone or with co-thickeners. They are noted as excellent mid- and high-shear viscosity builders. Unlike cellulosic or polyacrylic thickeners, HEUR thickeners do not affect the water sensitivity of a formulation.

Non-Associative Modification Mechanism
The most prevalent non-associative mechanism is hydrodynamic volume exclusion. This mechanism is simply based on the fact that certain substances in the solution occupy some volume of the solution, thereby excluding the possibility of any other substance occupying the same space. As more of the substance is added, less volume is available within the solution, resulting in increased solution viscosity.
ASEs use this mechanism in a unique way. Within the surfactant stable thickener product, there exists a tightly coiled water-insoluble polymer typically of molecular weight in the 200,000 to 1.5 million range. Addition of alkali to the polymer emulsion results in neutralization of the carboxylic acid groups, generating an anionic charge at the acid sites along the polymer chain. The like charges repel one another resulting in swelling and uncoiling of the polymer.
ASEs and cellulosics rheology modifiers are the most common chemistries utilizing non-associative mechanism and they are also referred as non-associative modifiers.
#1. Alkali Swellable or Soluble Emulsions (ASEs)
ASEs are based on homopolymers of methacrylic acid and copolymer of methacrylic acid and methacrylic esters, and maleic acid, among others. They are generally available as free-flowing liquids of 20-30% solids in water and can easily be added immediately before or during manufacturing processes. Alkali swellable or soluble emulsions (ASEs) rheology modifiers increase viscosity by the swelling and uncoiling of the polymer modifier when exposed to an alkaline environment.
There are a number of ASE types available and each enhances viscosity development and flow properties. They provide highly predictable rheological properties.
Compared to cellulosics (discussed below), they provide improved flow, have better resistance to microbial degradation, and are more economical to use. As a result, they are sometimes used as full or partial replacements for HEC.
#2. Cellulosic Thickeners
Cellulosic thickeners can be used to thicken and stabilize water-borne adhesives. They are generally simple to use and tolerant to small formulation changes.
With a range of molecular weights and thickening efficiencies, cellulosic polymers help control flow during formulation and coating. They resist sagging once the adhesive is applied. In addition, the products’ non-ionic nature provides wide formulation latitude and offers good heat and shelf-life stability.
When choosing one of these products, the formulator generally must consider:
-
Initial viscosity (as these products come in grades that vary from 2 to 50,000 cps at several percent concentration in water),
-
Solubility time, and
-
Thickening efficiency.
The higher viscosity products generally find use in caulk and sealant formulations.
Hydroxyethyl cellulose (HEC) is the most commonly used cellulosic thickener.
- It is a non-ionic polymer made by swelling cellulose with NaOH and reacting with ethylene oxide.
- HEC is soluble in hot or cold water and gives clear, colorless, neutral pH solutions.
- Solutions are pseudoplastic (viscosity decreases with shear rate and lacks a yield value).
- HEC solutions are compatible with most water-soluble gums and resins.
- They are sometimes used with sodium carboxymethyl cellulose or sodium alignate because the mixtures provide synergistic thickening characteristics.
More chemistries are listed in the table below.
| Cellulosic Chemistry |
Rheology Characteristics |
| Hydroxyethyl cellulose |
Solutions are pseudoplastic with no yield value, and they show a reversible decrease in viscosity at elevated temperatures. |
Methylcellulose
Methyl Hydroxypropylmethyl cellulose |
Solutions are pseudoplastic and have characteristic gelation temperatures between 50 and 85°C. The gels are reversible and return to fluidity on cooling. Non-gelled solutions lack yield value. |
| Sodium carboxymethyl cellulose |
Most solutions are pseudoplastic with little or no yield value. All solutions show a reversible decrease in viscosity at elevated temperatures.
|
Commonly Used Cellulosic Rheological Additives
Based on the thickening mechanism as well as the rheological profiles provided by these thickeners, it is possible to select the thickener dependent on the shear rates that are expected. In the figure below, rheological profiles are indicated for each thickener.
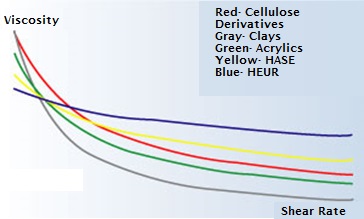 Indicative Rheological Profiles (Viscosity-shearRate) of Several Rheology Modifiers
Indicative Rheological Profiles (Viscosity-shearRate) of Several Rheology Modifiers
in a Water-borne Latex System[3]
The following table lists the advantages and disadvantages of each rheology modifier chemistry we discussed above.
| Rheology Modifier |
Advantages |
Disadvantages |
| Cellulose Rheology Modifiers |
- Wide range of applications and formulation latitude (non-ionic)
- Solids (difficult compounding)
- Shear-thinning
- Open time control by water retention
- Sag control
- Good heat and shelf-life stability
|
- Low efficiency
- Poor moisture resistance of dried adhesive film
- Degradation due to microbes
- Roller splattering
- Poor flow and leveling
|
| Alkali-swellable Acrylic Emulsions (ASE) |
- Strong shear thinning
- Flowable liquid (easy compounding)
- Resistant to microbial degradation
- Not sensitive to pH
- Good spray properties
- Lower cost than cellulosics
- Anti-settling and anti-sag
- Flowable liquid (easy compounding)
|
- Moderate efficiency
- Limited film build
- Moderate water sensitivity
|
| Hydrophobically Modified Alkali-swellable Acrylic Emulsions (HASE) |
- Strong shear thinning
- Greater rheology control over a broad range of shear rate
- High film build
- Sag resistant
- Resistant to microbial degradation
- Flowable liquid (easy compounding)
- Very efficient, low formulation costs
- Good moisture resistance of dried film
|
- pH sensitivity
- Greater skill and care necessary for selecting the proper modifier
|
| Hydrophobically Modified, Ethoxylated Urethane Resins (HEURs) |
- Excellent mid and high shear viscosity
- Resistant to microbial degradation
- Non-ionic and not sensitive to pH
- Flowable liquid (easy compounding)
- Very efficient
- Depending on type can provide Newtonian or pseudoplastic characteristics
|
- Lesser degree of sag control
- Greater skill and care necessary for selecting the proper modifier
- More expensive cellulosics, ASE, or HASE
|
Advantages and Disadvantages of Common Rheology Modifiers Used
in Water-borne Adhesive Formulations
Natural Gums (Polysaccharides) as Rheology Modifiers
After cellulosic materials, other naturally occurring polymers that are mostly used to thicken adhesive and sealant systems include polysaccharide gums. These naturally occurring thickeners are generally water soluble.
Natural gums are more commonly used as rheology modifiers in food products, cosmetics, and other personal care products due to their safe and non-toxic nature. However, they are also used in water-borne adhesives, sealants and coatings to achieve viscosity modification and stability during storage. These natural products include xanthan gum, guar gum, and others.
Their mechanism of stability depends on the nature of their macromolecules. Some adsorb on the polymer-water interface while others modify only the aqueous phase viscosity. The
table below lists characteristics of natural gums that are commonly used as rheology modifiers in water-borne systems.
| Natural Gum |
General Characteristics |
Rheological Characteristics |
| Xanthan gum |
- Soluble in hot or cold water
- Provides hazy, neutral pH solutions
- More tolerant of acids and bases than other organic gums
- More resistant to shear, heat, bacteria, and UV than most gums
|
- Typically used in the 1500 to 2500 cps range at 1%
- Pseudoplastic and shear-thinning
- Good viscosity stability at elevated temperatures
- Possess excellent yield values
|
| Hydroxypropyl guar gum |
- Non-ionic
- Soluble in hot or cold water and gives clear solutions
- Good resistance to bacteria and enzyme degradation
|
- High viscosity, pseudoplastic solutions that show a reversible decrease in viscosity at elevated temperatures
- No yield value
- Good viscosity stability (2-13 pH)
|
| Gum arabic |
- Anionic
- Soluble in hot or cold water and gives clear solutions at neutral to acidic pH
- Compatible with moderate amounts of salts, acids, bases and with most water soluble thickener
- Stable solutions over a wide pH range
|
- Very low viscosity with concentration up to 50% in water
- Solutions that show a reversible decrease in viscosity at elevated temperatures
- Shows a yield value at sufficient concentrations
- Viscosity peaks at pH 6, dropping sharply below pH 5 and above pH 7
|
| Gum tragacanth |
- Anionic
- Produces hazy, surface reactive solution of slightly acidic pH in hot or cold water
- Lowers surface and interfacial tension
- Effective emulsion stabilizer
- Available in grades of varying quality
- Compatible with most water soluble thickeners
- Somewhat resistant to biodegradation
|
- Typically used in the 300 to 3000 cps range at 1%
- Pseudoplastic solutions show a reversible decrease in viscosity at elevated temperatures
- Good yield value
- Solutions are stable between pH 2 and pH 11 with some loss in viscosity at pH less than 5.
|
Characteristics of Commonly Used Natural Gum Rheology Modifiers
Other Rheology Modifiers for Water-borne Systems
Several other natural and synthetic viscosity thickeners are available for the water-borne adhesive formulations. These are summarized in the table that follows.
| Thickeners Chemistry |
Rheology Charateristics |
| Sodium salt acrylic polymer |
Liquid dispersion polymers are made from sodium salt acrylic polymer. These have superior electrolyte tolerance. They are available in powder form and high solids (60%) emulsions. They are effective across a wide range of water-borne systems and applications. |
| Polyvinyl pyrolidone |
These polymers are readily water soluble. Thickening is not affected by pH. They provide Newtonian flow behavior. |
| Styrene butadiene rubber (SBR) |
These are prepared from emulsion copolymerization of styrene and butadiene. In addition to viscosity building, SBR additives promote adhesion. |
| Clays |
Clays and modified clays are mainly based on bentonite, naturally occurring smectite clay. Hectorite is composed of silicate sheets which delaminate in water to provide a 3-dimensional structure and viscosity thickening. These are widely used as rheological additives in adhesives and sealants. |
| Silicates and other mineral thickeners |
Mineral additives have been used for many years as functional extenders and fillers in adhesives and sealants. These include kaolin (hydrated aluminum silicate), talc (magnesium silicate), and attapulgite (hydrated magnesium aluminum silicate) additives. Kaolin and talc are considered to be viscosity thickeners; whereas, attapulgite is more of a conventional thixotrope. They are considered to be very cost effective rheological additives.
|
Viscosity Thickeners Other than Cellulosics, ASEs, HASEs, and HEURs
Organic Rheology Modifiers for Solvent-based & Solvent-less Systems
Organic Rheology Modifiers for Solvent-based & Solvent-less Systems
Castor Oil Derivatives
Hydrogenated castor oils or castor oil derivatives are widely used thickeners for non-aqueous systems. Much like cellulose, castor oil has hydroxyl groups which provide good interfacial characteristics for rheology modification.
Castor oil has double bonds like other oils; however, with castor oil most substitutions occur at the hydroxyl functional group, allowing derivatives with numerous properties. The best thickening is achieved when the hydrogenated castor oil is only partly dissolved in the formulation. The organic solvents greatly affect the thickening efficiency of these oils.
The optimum temperature for incorporation is between 35°C and 70°C. They may lose their rheological properties at high temperatures and/or when dissolved in solvents such as xylene after long dwell times. Hydrogenated castor oils are available as a powder or a paste.
Modified Polyurea
Modified urea compounds can be used as very powerful liquid rheology additives. Their rheological impact is directly related to the specific chemical modifications that can be made to the polyurea molecule (e.g., end groups of varying polarities, medium polarity segments, or highly polar structures).
They function by two different thickening mechanisms:
- Hydrogen-bonding between urea and urea groups of the additive and
- Association of the additive with the binder
Modified polyureas have exceptional resistance to settling during storage, along with good sag resistance. Some urea-type additives used in combination with conventional rheology modifiers, such as fumed silica, show synergistic effects.
Polyamides
Polyamide rheology modifiers are also available in a variety of chemical compositions. The thickening effect is partly explained by chelating and partly by the formation of micelles as a result of hydrophilic and hydrophobic end groups. The formation of hydrogen bonds also appears to play a role. These interactions typically result in a steep shear-thinning viscosity profile.
Polyamides require high incorporation temperatures. For some polyamides, this may be as high as 150°C.
A major problem with polyamide thickeners is the negative effect these additives may have on adhesion. The polyamide molecules can migrate to an interface causing a weak boundary layer.
Calcium Sulfonates
Calcium sulfonates achieve their increased viscosity through the microstructures that are formed. A temperature-stable gel structure is strongly shear-thinning and has good sag resistance.
-
Advantage – Easy incorporation into a formulation since they are supplied in
liquid form
- Disadvantage – High alkalinity
Select Suitable Rheology Modifier for Your Solvent-based System »
Inorganic Rheology Modifiers
Inorganic Rheology Modifiers
Fumed Silica (Amorphous Silicon Dioxide)
Fumed silica is a versatile, efficient rheology additive used in adhesive and sealant formulations for flow control and thixotropy. It has long been the dominant thixotrope employed in the adhesive and sealant industry.
Key Characteristics of Fumed Silica
- It is typically available with sizes in the 7-40 nanometer range and surface areas ranging from 50 to 380 m2/g.
- Unlike precipitated silica, fumed silica has no internal surface area. The specific gravity of fumed silica is approximately 2.2.
- Because of its high surface area to weight ratio, formulations generally require only a little fumed silica (1-5% by weight) to achieve thixotropic properties.
- The surface chemistry of fumed silica is extremely important because of its influence on the rheological behavior of the formulation. Thus, fumed silica grades are generally characterized by their surface area and whether they are hydrophilic (standard grade) or hydrophobic.
- Hydrophilic silica: Most effective in non-polar and medium polar media.
- Hydrophobic silica: In medium polar to polar media, hydrophobic fumed silica is a more efficient thickening agent and is generally preferred. Also noted for providing superior moisture resistance in adhesives, sealants, and coatings.
The performance of hydrophilic fumed silica is often improved by adding a polar substance such as ethylene glycol, glycerin, or some secondary amines to the formulation.
- The thixotropic characteristics provided by fumed silica are due to its ability to develop a loosely woven, lattice-like network by hydrogen bonding between particles. This network raises the apparent viscosity of the system, increases the cohesive forces, and contributes to the suspension of solid components.
- Because the hydrogen bonds themselves are relatively weak, they are easily disrupted through the action of an applied stress or shearing force and quickly reformed when the stress or shearing force is removed.
|
Important Parameters for the Performance of Fumed Silica Systems
- Nature of the system (polarity)
- Concentration of the silica
- Grade of silica used (particle size, surface area, density, surface chemistry, etc.)
- Degree of dispersion
- Presence of additives in the formulation other than the fumed silica
|
At times the results of the formulation are less than expected because these factors are not considered or understood relative to the final rheological properties. For example, proper dispersion can maximize the efficiency of both hydrophilic and hydrophobic fumed silica.
To ensure proper dispersion, addition of silica to the formulation in the right sequence, as well as effectiveness of the dispersion equipment becomes very important.
High shear mixing equipment will improve the efficiency of fumed silica in the formation. In most cases, silica should be added to the resin directly or to the more viscous part of the formulation with as little solvent or diluent as possible. Incorporating the silica before adding any fillers or pigments assures homogeneous distribution.
Dispersing the silica in a concentrated base, or master batching, provides optimum efficiency and stability.
Features of Fumed Silica with Different Adhesive Systems
Fumed silica is often used in 100% solids, liquid polymers.
- With epoxy adhesives and sealants only a few percent by weight of the additive will eliminate common problems such as slumping and separation.
-
It raises the effective viscosity of the base resin to prevent other components from settling while the extrudability or spreadability is unaffected.
- It provides a surface that is free of texture which is important in architectural grade paints and sealants.
-
It is also effective in butyl and urethane sealants.
- With polyurethane systems, the thixotropic gels formed with ordinary fumed silica are at times unstable when shipped and stored due to the reaction of the surface hydroxyls with the isocyanate groups. However, this problem can be eliminated by using silane-treated fumed silica or adding small percentages of polyoxypropylene prepolymer[6] to the formulation.
-
Fumed silica has also been used in pressure-sensitive and
hot melt adhesives to change the rheological properties and to enhance physical properties.
-
The table below illustrates the enhanced properties obtained with the use of fumed silica in a styrene-butadiene block copolymer-based pressure-sensitive adhesive without the loss of
adhesion or tack. The temperature resistance, tensile, and elongation values improve with as
little as 3% addition of the silica.
- In hot melt systems, fumed silica has been
incorporated into resins such as polystyrene blocked copolymer and butyl rubber. 5% of added fumed silica (provided that it is dispersed properly) provides an increase in shore
hardness, tensile strength, and elongation without the loss of peel strength.
|
|
Without Fumed Silica |
With Fumed Silica
|
| Film thickness, mils |
1.5
|
1.5
|
| Specific gravity |
0.93
|
0.93
|
| Rolling ball tack, cm |
0.5
|
0.5
|
| Probe tack, kg |
2.0
|
2.1
|
| Shear adhesive failure temperature, °F |
190
|
190
|
| Tensile strength, psi |
700
|
780
|
| Elongation at break, % |
660
|
680
|
| Hardness, Shore A |
61
|
68
|
| Peel temperature, °F |
150
|
190
|
| Creep, 240°F hold, mins |
100
|
180
|
Properties of Pressure Sensitive Adhesive with and without FumedSilica. (Adhesive based on Kraton copolymer.)[7]
Precipitated Calcium Carbonate
Precipitated calcium carbonate (CaCO3) functions as a thixotrope as well as being a low-cost extender (often used in the 40-50% by weight range) and reinforcement.
- Ultrafine (< 100 nanometer) precipitated calcium carbonate provides the greatest efficiency.
-
These have surface areas from 15 to 30 m2/g.
- CaCO3 is hydrophilic, and as a result, it has a tendency to agglomerate in organic polymers and
plasticizers.
- Precipitated calcium carbonates are generally surface treated to render them hydrophobic and to improve their dispensability in hydrophobic systems.
Calcium carbonate filler is generally employed to impart thixotropic properties and as an extender. However, improvements in physical properties such as tensile strength, tear, and elongation have been noticed as a function of surface area. This effect is noted more so with the coated CaCO3 products. Basic elastomeric reinforcement rules apply in determining the physical properties of CaCO3 filled sealants.
Surface treatment also improves adhesion to the polymer matrix and resulting physical properties. The combination of particle size and surface treatment is critical in the selection of precipitated calcium carbonate fillers to obtain desired properties. Often graded combinations of ultrafine precipitated CaCO3 and larger CaCO3 particles are used for optimum properties and value.
Conventional surface treatment of CaCO3 is with fatty acids; however, the use of different types of acids yields subtle differences in filler wettability and polymer compatibility. Functional precipitated calcium carbonate fillers can be developed to impart response specific to the base polymer in an adhesive or sealant.
Low modulus sealant standards can be met with stearine treatment, which decouples the precipitated calcium carbonate from the polymer but helps the filler wet-out and disperse into the polymer during the compounding operation.[8]
Other Mineral Additives
Conventional mineral additives have been used for many years as functional extenders and fillers in adhesives and sealants. These include:
#1 Kaolin
Kaolin is a commonly used inexpensive filler used primarily as an extender in adhesive and
sealant formulations. The cost of the adhesive is reduced because the kaolin addition
increases the product's volume.
Depending on the grade, kaolin can also:
- Control viscosity to prevent drip or sag
- Improve surface smoothness
- Provide reinforcement, improve wet tack development
- Speed dry or set time primarily by increasing solids content
- Reduce shrinkage
The finer particle sizes build viscosity more efficiently and provide better reinforcement than their coarser counterparts. Fine grades of kaolin have a particle size of 0.5 microns or less. Kaolin has a hexagonal particle shape with the finer particles occurring as platelets and coarser particles forming stacks or books of platelets. It is relatively soft and is a non-abrasive filler.
As with other solid rheological additives, the key parameters in choosing a particular grade are particle size, shape, and particle distribution. Kaolin is also available in hydrous and calcinated (where dehydroxylation is used to remove water) grades. The calcinated grades have a more porous particle shape. The table below shows common applications for these types of kaolin.
|
Hydrous Kaolin
|
Calcined Kaolin
|
| Applications |
- Paper and packaging
-
General-purpose
-
Caulking compounds
-
Mastics
-
Sealants
|
- Automotive sealants
-
Construction
|
| Systems |
- Starch
-
Protein
-
Latex emulsion
-
Polybutene
-
Butyl
-
Other polymeric
|
- Urethane (singe component and moisture
cure)
-
Specialty elastomer
|
| Loading levels |
- Adhesives: 5-25%
-
Mastics: 25-45%
- Sealants: 5-20%
|
|
| Benefits |
- Inert good cure rate
-
Reinforcement
-
Reduce titanium dioxide
-
Low moisture
-
Chemical and acid resistance
|
- Reduce adhesive cost
-
Reinforcement
-
Improve tack and penetration
-
Reduce dry time and shrinkage
-
Viscosity control
-
Chemical and acid resistance
|
Uses for Hydrous and Calcined Kaolin Clay[9]
Special surface modifications are available to further improve reinforcement. The objective of the surface treatment is to increase filler loading and/or improve physical properties without loss of rheological characteristics.
A variety of surface-modified kaolins have been introduced including clays treated with silane, titanate, polyester, and metal hydroxide. Silane-treated kaolin is used in applications requiring maximum aging characteristics in the service environment.
#2 Bentonite
Bentonite is a colloidal clay that is both hydrophilic and organophilic.
- It is water swelling with some types of clay absorbing as much as five times its own weight in water.
- It is used in emulsions, adhesives, and sealants.
- It is a gritty, abrasive white particle filler.
- Once hydrated, most clays of this type form an alkaline dispersion.
- A macroscopic particle of bentonite is composed of many thousands of stacked and/or overlapped submicroscopic flakes.
- When the clay and water are mixed, water penetrates the area between the flakes, forcing them apart.
- In its natural state, the hydroxy groups of the clay tend to cluster in water setting up a network.
- It produces thixotropic-like dispersions. To make them effective in organic materials, the surface of the clay is treated with an aliphatic tertiary amine. In this case, the uncoiling of the organic chains sets up the
network.
Adhesives formulated with bentonite generally have a rheology profile that is less shear-thinning or more Newtonian than other rheological additives.
 Bentonite formulations are not classical thixotropic materials with a defined yield point. They, therefore, are more suitable for thin-film applications such as pressure sensitive or packaging adhesives.
Bentonite formulations are not classical thixotropic materials with a defined yield point. They, therefore, are more suitable for thin-film applications such as pressure sensitive or packaging adhesives.
Often combinations of clay-based thickeners with water-based thickeners, such as polysaccharides and alkali-swellable emulsions often provide synergistic performance particularly when it comes to sag and suspension control.
BENTONE® and BENAQUA® are commercial (Elementis) grades of organoclay that is used as gelling agents for emulsion paints and adhesives.
-
They provide shear-thinning when spreading and spraying, fast viscosity recovery
for good sag and slump control.
- They are compatible with most resin systems.[10] However, certain
types of amine-coated clays have been found to destabilize single component polyurethanes.
#3. Talc
Talc is also often used as an extender in adhesives in
sealants.
- It has flow control properties.
- It is used in higher solids, high viscosity
applications such as caulking compounds, automotive putties, mastics and sealants.
- It is a
hydrophobic and organophilic material.
- Talcs are either platy or acicular in particle shape.
-
Thin platelet particles have aspect ratios varying from 20:1 to 5:1.
- Coarse particle sizes
(10-75 microns) are commonly used in these applications at loading levels of 5-30%.
- Fine talcs
(1-10 microns) are more expensive and require intensive dispersion processes.
- Platy grades
enhance barrier properties and air, water, and chemical resistance.
- Polymers filled with platy talc exhibit higher stiffness,
tensile strength, and creep resistance than do polymers filled with standard particulate
fillers. These properties are maintained at both ambient and elevated temperatures.
- Surface treatments for talc particles, include magnesium and zinc stearates, silanes, and titanates.
#4 Attapulgite
Attapulgite is a very cost-effective thixotrope.[11] However, unlike kaolin or talc it is not used at high loadings and, thus, is not considered as an extender. Attapulgite consists of acicular-shaped particles with a size of about 0.1 micron.
Thickening occurs upon the mutual separation of individual particles as the porous mineral surface is exposed to the surrounding medium and forms stable colloids. Cationic surfactants often enhance the performance of attapulgite thixotropes in organic systems.
Attapulgites are categorized as either sorbent or colloidal.
- The sorbent grades are generally used as oil adsorbents and purifiers.
- The
colloidal grades are used as thixotropes and rheological modifiers in both water-based and
solvent-based polymer systems.
They are commonly used in coatings, asphalt cutback, and tape
joint compounds as well as adhesives and sealants.
Water-based applications include polyvinyl acetate and vinyl emulsion systems. The inorganic attapulgite is not wate- soluble as are other cellulose and organic thickeners used in water-based systems. Therefore, formulations made with attapulgite tend to be less water sensitive.
Typical usage levels range from 2-8% by weight. Attapulgite particles are easy to disperse and commonly added to the formulation with other dry components. At levels ranging from 10-20% attapulgite is considered to be a partial asbestos replacement in polymeric-based sealants for the construction and automotive industries.
Kaolin and talc are considered to be viscosity thickeners; whereas, attapulgite is more of a conventional thixotrope.
They are considered to be very cost-effective rheological additives.
Fibrous Rheology Modifiers
Several types of inorganic and organic fibers are used as thixotropic additives in both adhesive and sealant formulations. These are chiefly cellulose, glass, polyolefin, and aramid fibers. They are primarily used in solvent-less adhesive and sealant systems, but they can also be used in water-borne and solvent-based systems, especially sealants.
A fiber that exhibits good thixotropy usually has several key characteristics including:
- The ability to interact with the polymer matrix via dipolar and / or dispersion interactions
- Ability to achieve polymeric entanglement
- The presence of branch-like fibrils on the fiber which increase the surface area and promote rheological effects, and
- A high surface area with good surface activity.
It is interesting to note that these characteristics also are necessary to impart physical reinforcement and cohesive strength improvement to the adhesive or sealant.
Cellulose fibers (e.g., Interfibe RT Cellulose Fiber from Interfibe) are perhaps the best-accepted thixotrope of this group. This material provides:
- Good thixotropy
- Reinforcement of the bulk polymer
- Minimal surface texture, and
- Low cost
Cellulose fibers are claimed to provide greater formulation latitude than the other thixotropes at a reduced cost. They are easy to disperse in water and oil-based solutions. The dispersement in water solutions is assisted by the application of nonionic surfactants during the manufacturing process. The resulting formulations are able to hold pigments and extenders in suspension.
Polyolefin (Spectra, Honeywell-Allied Signal) and aramid (Kevlar, DuPont) fibers have much lower surface areas and polarities than asbestos fibers and do not bond well with all polymeric matrix resins. However, aramid fibers have been proposed as a replacement for asbestos or fumed silica.[5]
-
Advantages: Aramid fibers provide better sag resistance than fumed silica.
-
Disadvantages: Aramid fibers are very expensive, and they increase modulus by as much as 2.5 times that of fumed silica.
Selecting Rheology Modifiers
Selecting Rheology Modifiers
Unfortunately, there is no universal rheology modifier or thickener, and the selection of the optimal additive and its incorporation into an adhesive formulation requires special consideration.
Selection of the proper rheology modifier can be a complex process because of the following factors.
- The required rheology profile may vary drastically during the adhesive's lifetime, and
- There are many modifiers in the market and none represents a universal solution.
Selection Relative to Adhesive Type and Form of Rheology Modifier
The table below provides a guide as to the rheology modifiers that have been typically used with various adhesive systems. These commercial rheology modifiers are listed and maintained in the SpecialChem material selector database.
Since there is a broad range of rheology modifiers and chemistries available, the formulator should discuss the optimal procedure for specific formulations with the supplier of the modifiers.
|
|
Alkali-solubles (associative)
|
Alkali-swellable acrylic polymers (ASE/HASE)
|
Carboxyl & polycarboxylic acids & acid amides
|
Cellulosics
|
Metal
organic
gellants
|
Minerals
|
Natural
gums
|
Organo
clays
|
Urethanes (HEUR)
|
|
Acrylic and acrylic copolymers
|
✓
|
✓
|
✓
|
✓
|
|
✓
|
|
✓
|
✓
|
|
Aminoplasts / phenoplasts
|
|
|
|
|
|
✓
|
|
|
|
|
Epoxies
|
|
✓
|
✓
|
|
|
✓
|
|
✓
|
|
|
Ethylene co-terpolymer emulsions
|
✓
|
|
|
✓
|
|
|
|
|
|
|
Ethylene co-terpolymer solids
|
|
|
|
|
✓
|
✓
|
|
|
|
|
Natural rubbers
|
|
|
|
✓
|
|
✓
|
|
|
|
|
Polyamides
|
|
|
|
|
|
✓
|
|
✓
|
|
|
Polychloride covinyls
|
|
✓
|
|
|
|
✓
|
|
|
|
|
Polyesters
|
|
✓
|
✓
|
|
|
✓
|
|
✓
|
|
|
Polysulfides
|
|
|
|
|
|
✓
|
|
✓
|
|
|
Polyurethanes
|
✓
|
✓
|
✓
|
|
|
✓
|
|
✓
|
✓
|
|
Polyvinyl acetate emulsions and derivatives
|
✓
|
|
|
✓
|
|
✓
|
✓
|
|
✓
|
|
Polyvinyl alcohol
|
|
|
|
✓
|
|
✓
|
|
✓
|
|
|
Silicones
|
|
|
|
|
✓
|
✓
|
|
✓
|
|
|
Silyl-modified polymers
|
|
|
|
|
|
✓
|
|
✓
|
|
|
Styrene copolymers
|
|
|
|
|
|
✓
|
|
✓
|
|
|
Synthetic rubbers
|
✓
|
✓
|
|
|
✓
|
✓
|
|
|
|
Rheology Modifiers for Specific Adhesive Chemistries
These rheology modifiers are supplied in several forms. The choice of form is mainly dependent on the compounding processes used by the formulator and whether it is water-borne,
solvent-based, or solvent-less system.
The table below shows the forms that are typically available for common rheology modifiers that are described in the SpecialChem product selector. Again, since there is a broad range of rheology modifiers and forms available, the formulator should discuss the optimal form for specific formulations with the supplier of the modifiers.
| |
Alkali-solubles (associative)
|
Alkali-swellable acrylic polymers (ASE/HASE)
|
Carboxyl & polycarboxylic acids & acid amides
|
Cellulosics
|
Metal organic gellants
|
Minerals
|
Natural
gums
|
Organoclays
|
Urethanes
|
|
Beads
|
|
|
|
|
|
✓
|
|
|
|
|
Crystals
|
|
|
|
✓
|
|
✓
|
|
|
|
|
Dispersion
|
|
|
✓
|
|
|
✓
|
|
|
|
|
Emulsion
|
✓
|
✓
|
✓
|
|
|
|
|
|
|
|
Flakes
|
|
|
|
|
|
✓
|
|
✓
|
|
|
Gel
|
|
|
|
|
✓
|
|
|
|
|
|
Granules
|
|
|
|
✓
|
|
✓
|
|
✓
|
|
|
Liquid
|
✓
|
✓
|
✓
|
✓
|
✓
|
|
|
|
✓
|
|
Paste
|
|
|
|
|
✓
|
|
✓
|
|
|
|
Powder
|
|
✓
|
✓
|
✓
|
|
✓
|
✓
|
✓
|
✓
|
|
Solid
|
|
|
|
|
|
|
✓
|
|
|
Forms of Common Rheology Modifiers
Selection Relative to Performance Properties
Considerations for selecting a rheology modifier include the criteria listed in the table below. The comparative properties of the various rheology modifiers are generally provided in the discussions above. These can be condensed into the following criteria for both water-borne and
solvent-based formulations:
- Rheological requirements of the liquid formulation (during all stages of its product life)
- Ease of incorporation into the formulation
- Physical properties of the adhesive coating or sealant after it is immediately applied
- Permanence and durability after it is applied and during its service life
- Total cost of the formulation (materials, processing, energy, waste, etc.).
|
Required Rheological Properties
|
Required Performance as a Liquid Coating
|
Required Performance Properties in a Joint
|
- Sag resistance
- Flow and leveling
- Application performance (spray, brush, roller, etc.)
- Stability and resistance to sedimentation
- Ease of incorporation (mixing requirements, pumpability, etc.)
|
- Appearance (uniformity, transparency, color stability, etc.)
- In-can stability (bio-stability and resistance to separation)
|
- Moisture resistance
- Durability
- Adhesion
- Minimal negative effect on other physical properties
|
Selection Considerations for Rheology Modifiers in Adhesives and Sealants
Formulation Guidelines