Advantages and Disadvantages of Surfactants
Advantages and Disadvantages of Surfactants
Surfactants are used in both waterborne and solvent-based adhesive formulations. In solvent-based systems, their primary use is as dispersing agents.
In adhesive formulations, surfactants are used to change many end properties. They are used in the adhesive formulations to provide the following characteristics without negatively impacting adhesion.
- Reduce surface tension to extremely low values for improved substrate wetting
- Help to avoid defects occurring in the adhesive coating - especially on contaminated or difficult surfaces
- Improve flow
- Promote wetting and dispersion of additives
- Optimize stability of pigments and latex particles
- Overcome foaming issues
They are also used by the latex raw material manufacturing to emulsify polymers in their liquid state and to provide the following characteristics:
- Micelles formulation, where the polymerization reaction takes place
- Protection of the polymer particles from agglomeration due to external stress (e.g., temperature during storage, high shear during application, etc.)
- Significant reduction in surface tension
- Prevent excessive foaming
Drawbacks of Using Surfactants in Adhesive Formulations
The major disadvantage of using surfactants is that they generally increase the water sensitivity of the adhesive. The surfactant also may not stabilize a latex in difficult situations, such as when the product is applied at high speed or when mixed with other systems.
Due to these factors and the thousands of surfactants that are commercially available, the selection process is difficult. Also, the formulator often needs to turn to trial-and-error.
Let's discuss each and every factor you should consider while selecting a suitable surfactant for your formulation in detail.
Classification of Surfactants
Classification of Surfactants
To understand how surfactants operate and to select a surfactant for a specific purpose, it is necessary to classify surfactants according to their structural features. From the commercial point of view, surfactants are often classified according to their use. However, this is not very efficient because many surfactants have several uses.
For example, surfactants can be used as emulsifiers in one application and as wetting agents, dispersants, or stabilizers in another. A more accepted approach to classifying surfactants is by their structure and chemistry.
Structure of a Surfactant
A surfactant molecule consists of two key groups in its structure.
- Polar hydrophilic head groups – It makes the surfactant soluble in polar solvents such as water.
- Non-polar hydrophobic tail groups – It makes the surfactant soluble in non-polar solvents and oil.
The relative sizes and shapes of the hydrophobic and hydrophilic parts of the surfactant molecule determine many of its properties.
The hydrophobic group in the surfactant structure is made up of hydrocarbon chains, fluorocarbon chains, and a combination of fluorocarbon and hydrocarbon chains or silicone chains.
However, surfactants are also characterized by the chemical structure of their hydrophilic groups, either ionic or non-ionic. This is depicted in the figure below.
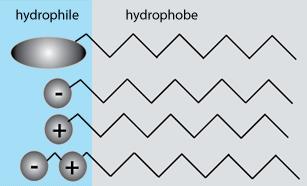 Surfactant classification according to the composition of their head:
Surfactant classification according to the composition of their head:
from top to down respectively non-ionic, anionic, cationic, and amphoteric
Ionic surfactants can, unlike non-ionic surfactants, dissociate into ions in an aqueous medium.
The hydrophobic part can belong to a negative or positive ion.
Surfactants can generally be classified into four types:
- Anionic surfactants – The hydrophobic part is an anion, for example I-
- Cationic surfactants – The hydrophobic part is a cation, for example Na+
- Amphoteric surfactants – They have at least one anionic and one cationic group
- Non-ionic surfactants – They have neither positive nor negative charge
The hundreds of commercially available surfactants can be divided into the basic classes described above which makes the selection process much easier. The most important classes for adhesive and emulsion formulations are non-ionic surfactants and anionic surfactants.
Let's take a deeper look at these classes.
Anionic surfactants
Anionic surfactants contain negatively charged functional groups at their head. Examples of anionic surfactants are:
- Sulfates
- Carboxylates, and
- Phosphate esters
These are the most common surfactant in general use (detergents, cleaners, etc.) and many find use in emulsion polymerization. This is because of their affinity for hydrogen bonding with the aqueous medium.
Cationic surfactants
Cationic surfactants contain positively charged functional groups at their head. Cationic surfactants are typically amine derivatives, such as quaternary ammonium compounds. Most of these surfactants are used as anti-microbials and anti-fungals. Cationic surfactants are less commonly used in adhesive formulations because of their certain limitations, such as:
- High cost
- Inefficient emulsifying capability, and
- Undesirable effects on initiator decomposition
Cationic surfactants are avoided for being applied in waterborne systems. However, they are successfully used in solvent-borne systems, for instance to support the wetting and dispersing process.
Non-ionic Surfactants
Non-ionic surfactants (alcohol ethoxylate, EO/PO types, etc.) can be differentiated from ionic surfactants in important ways.
- They do not dissociate into ions.
- Since they do not dissociate into ions in water, they are less sensitive to electrolytes and pH changes.
- They are soluble in acid and alkaline medium and are compatible with ionic and amphoteric species.
- Unlike ionic surfactant, these surfactants are not preferentially adsorbed on charged surfaces.
- Their solubility decreases with increasing temperature and at the cloud point, the aqueous solution becomes turbid.
Non-ionic surfactants are commonly used in adhesive formulations. However, in emulsion polymerization, they are rarely used alone due to their inferior efficiency in creating stable emulsions in comparison to anionic surfactants. Because of this, non-ionic surfactants are usually used with anionic surfactants and impart a second method of colloidal stabilization. Latexes that require stability over large pH ranges use larger non-ionic to anionic surfactant ratios.
 The main classes of non-ionic surfactants are polyglycoether derivates such as:
The main classes of non-ionic surfactants are polyglycoether derivates such as:
- Alkyl- and alkyl-aryl polyethylene glycol ether (alkyl PEG)
- Polypropylene glycol ether, and
- Block copolymers of polyethylene glycol and polypropylene glycol
Many long chain alcohols exhibit non-ionic surfactant properties.
For non-ionic surfactants, the chemistry and length of the hydrophilic chain can be varied to modify what is called the hydrophile-lipophile balance (HLB) for the surfactant. This is the ratio based on molecular weight of the oil loving (hydrophilic) to the water loving portion (lipophile). HLB affects interfacial behavior and the stabilization of emulsions and is important in selecting a non-ionic surfactant.
Considering all industries, there are thousands of surfactants that are commercially available. Within the adhesives industry non-ionic surfactants along with specialty surfactants such as fluoro- and silicone-based surfactants are the most commonly used. Anionic surfactants are also used primarily in emulsion polymerization processes.
Descriptions of >400 surfactants are contained in the SpecialChem Adhesives Additives Database. They include the various types of surfactants that are shown in table below and focused on in the remainder of this guide.
| Anionic |
Cationic |
Non-ionic
|
Other
|
- Alkyl ether sulfates
- Phosphate esters
|
|
- Acetylenics
- Alcohol Ethoxylates
- Alcohols
- Alkyphenol ethoxylates
- Amides
- Amines
- Amine oxides
- Copolymers
- Fatty acids
- Nonylphenol ethoxylates
|
- Fluorosurfactants
- Silicone-based
|
Types of Surfactants
The description and example of commercial surfactants within types mentioned above is given in the table below,
| Surfactant Type |
Characteristics |
Example Product |
| Anionic Surfactants |
|
Alkyl ether sulfates |
- Used as co-emulsifier for the manufacture of emulsion polymers used
in adhesives
- Provides good mechanical stability and high resistance
to electrolytes
|
Alkylaryl polyglycol ether
|
|
Phosphate esters |
- VOC-free
- NPE-free
- Used as a highly hydrophilic emulsifier for
alkyd resin and tackifier resin emulsions for adhesives
- Offers high
efficiency, very good long term stability and high compatibility
- Can be used alone as single emulsifier or in combination with the
anionic surfactants
- Can be a UV curable additive
|
EO phosphoric acid ester
|
| Cationic Surfactants |
|
Alkylammonium salts |
- Used as emulsifier in adhesives
- Stabilizes the polymer dispersion
during polymerization and beyond - throughout the packaging,
transportation, mixing and application stages
|
Anionic ammonium lauryl sulfate
|
| Non-ionic Surfactants |
| Acetylenics |
- Low-VOC
- Low-foam
- Non-ionic wetting agent and surfactant
- Used in waterborne systems
- Possesses surfactant, low foam and low
- water-sensitivity properties
- Offers advantages such as enhanced dispersion quality and increased stability
|
Acetylenics |
|
Alcohol Ethoxylates |
- Acts as an emulsifier or surfactant and also as a dispersant or
dispersing agent
- Acts as a defoamer / antifoam agent and low foam
surfactant
- Shows excellent solvency, low foam characteristics,
chemical stability and other performance properties
|
POE-cetyl alcohol
|
|
Alcohols
|
- Acts as a non-ionic surfactant,
- Has good wetting and emulsifying properties
- Dispersant for organic and inorganic
particles
|
POE-tridecanol
|
|
Alkylphenol ethoxylates |
- Solvent free, proprietary surfactant blend, non-ionic/anionic and
wetting agent
- Used in adhesives
|
Alkylphenolethoxylate
|
|
Amides |
- Used as curing agent and surfactant for liquid epoxy resins in
waterborne and solvent-free sealants
|
Modified polyamide/epoxy adduct
|
|
Amine Oxides
|
- Provides emulsion stability and long term pH control for water
emulsions
- Neutralizes waterborne resins
- Improves long-term
stability
- Controls viscosity stability
- Can also aid in pigment
dispersion and acts as a thickener activator
|
Low toxicity trifunctional amine.
|
|
Amines |
- Acts as a very good cross-linker in PU systems improving water and
solvent resistance
- Low foaming water soluble surfactant used in
multiple applications as emulsifier or dispersant
|
Alkoxylated ethylene diamine
|
|
Copolymers |
- Acts as a defoamer / antifoam agent and low foam surfactant
- Shows excellent solvency, low foam characteristics, chemical
stability and other performance properties
- Can be used as a stabilizer in waterborne pressure sensitive
adhesives
- Enhances the colloidal stability of the latex and
improves dry film properties
- Reactive towards
vinyl unsaturated monomers
|
Linear EO/PO block copolymers
|
|
Fatty Acids
|
- Used as a pigment wetting and as a dispersing agent in water-based
adhesives and sealants
- Offers compatibility with:
- Water reducible
resins
- Pure acrylic
- Styrene acrylic
- Polyvinyl acetate and
copolymers
- Polyvinyl alcohol
- Vinyl acetate-ethylene pressure
polymers
- Casein and polymer emulsion-silicate combinations
|
Formulation of surfactant and modified fatty acids
|
|
Nonylphenol Ethoxylates
|
- Used in adhesives and emulsifier for the production of emulsion
polymers such as:
- Vinyl acetate
- Vinyl chloride
- Acrylates and
acrylic copolymers and
- Styrene copolymers
- Acts as a non-ionic surfactant
- Soluble in organic
polar solvents and water
- Versatile emulsion polymerization
surfactant and latex post-stabilizer
|
Sodium nonylphenolether sulphate
|
| Other Surfactant Chemistries |
|
Fluorosurfactants |
- Provides surface tensions as low as 15 dynes/cm in water at very low
concentrations
- Has excellent dynamic surface tension
properties
- Imparts
excellent wetting; spreading, leveling and flow control properties
on various types of water-based as well as solvent-based systems
- Typical uses include leveling and anti-static agents for adhesives
and caulks
- Applications are generally those in which typical
hydrocarbon surfactants are found to be inadequate
|
Short-chain perfluoro-based amphoteric fluorosurfactant
|
|
Silicone-based |
- Acts as a superwetting agent
- Contains no added:
- Hazardous air
pollutants (HAPs)
- Alkylphenol ethoxylates (APEs) and
- Volatile
organic compounds (VOCs)
- Provides a superior balance of
equilibrium, dynamic wetting, system compatibility and low foam when
compared to traditional siloxane surfactants
- Primarily recommended for high-quality waterborne adhesives
|
Siloxane based surfactant
|
|
Polyamide / Acid Ester Salts
|
- Used as a lead-free, high-efficiency activator for foam
compositions
- Can be used as an activator in plastic foams
- EPDM,
and PVC/nitrile sponge compounds
- Applications: closure sealing
gaskets for food containers
|
Stearic acid salt |
Description and Examples of Common Surfactants Used in Adhesives and Emulsions
Surface active adhesive additives are rarely mono-molecular products; they are mainly polymeric. These are preferred for reasons of providing:
- Best performance
- Best film integrity
- Low risk of being extracted from the dried film and minimal side effects
Working Mechanism of Surfactants
Working Mechanism of Surfactants
The term surfactant is the acronym of surface active agent. Surfactants are materials that lower the surface tension (or interfacial tension) between two liquids or between a liquid and a solid. The surface tension is defined as "the force which acts in a material to adapt the smallest possible surface under the set conditions".
In the general sense, any material that affects the interfacial surface tension, can be considered a surfactant. But in the practical sense, surfactants may act as wetting agents, emulsifiers, foaming agents, and dispersants. Surfactants reduce the surface tension of water by adsorbing at the liquid-air interface.
Surface activity is achieved when the number of carbon atoms in the hydrophobic tail is higher than 8.
- Surfactant activities are at a maximum if the carbon atoms are between 10 and 18 at which level a surfactant has good but limited solubility in water
- If the carbon number is less than 8 or more than 18, surfactant properties become minimal
- Below 8, a surfactant is very soluble and above 18, it is insoluble
Thus, the solubility and practical surfactant properties are somewhat related.
Micelle Formation
Many surfactants can also assemble in the bulk solution into aggregates. Such aggregates are known as “micelles”. The concentration at which surfactants begin to form micelles is known as the critical micelle concentration (CMC). When micelles form in water, their tails form a core that can encapsulate an oil droplet or polymer product, and their (ionic/polar) heads form an outer shell that maintains favorable contact with water. The formation of micelles leads to the stabilizing effect provided by surfactants in solution.
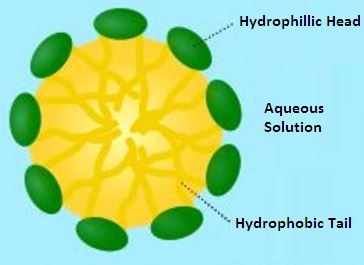 Micelles Around a Polymer Particle Provide Protection
Micelles Around a Polymer Particle Provide Protection
The hydrophobic tails form the core of the aggregate and the hydrophilic heads are in contact with the surrounding liquid. The shape of the aggregates depends on the chemical structure of the surfactants, namely the balance in size between hydrophilic head and hydrophobic tail. A measure of this is the HLB, hydrophilic-lipophilic balance.
Most waterborne adhesives are an emulsion of polymer particles dispersed in water. So surfactants are added by the emulsion manufacturer to lower interfacial tension and stabilize polymer particles to prevent demulsification.
What are the Properties Affected by Surfactants?
What are the Properties Affected by Surfactants?
Surfactants affect a wide array of physical properties in adhesive systems. Surfactants affect the behavior of the adhesive not only during the formulation and application process but also during the lifetime of the bonded joint.
For example, surfactants are used to stabilize the dispersion of polymer particles during emulsion polymerization. The addition of surfactants improves:
- Mechanical stability
- Freeze-thaw stability, and
- Shelf-life of paints
The addition of surfactants also allows the adhesive to coat and wet a surface more easily. This is because surfactants increase the wetting of a solution.
However, the addition of surfactants does not always have a positive effect on all properties. The water resistance of the coating can be decreased with surfactant addition. This is because surfactants can be very water-soluble and will easily migrate out of the adhesive bond during service. The type and amount of surfactant will determine which water properties are affected and the extent of the change.
Fluorocarbon and Silicone-based Surfactants
Fluorocarbon and silicone-based surfactants have a unique place in the surfactant industry. These surfactants in water and non-aqueous systems reduce the surface tension lower than the hydrocarbon chain surfactants. Both fluorocarbon and silicone chain surfactants have better thermal and chemical stability than hydrocarbons. Also, they provide excellent wetting for low-energy surfaces. However, due to their costs, these surfactants are used in limited applications.
Surfactants may be added before the emulsion polymerization process or to already manufactured emulsions to further improve their properties. A mixture of anionic and non-ionic emulsifiers is generally used.
Surface Active Properties of Surfactants
The principal surface active properties exhibited by surfactants are listed below1. Many surfactants possess a combination of these properties.
- Wetting
- Foaming / defoaming
- Emulsification / demulsification of emulsions
- Dispersion / aggregation of solids
- Solubilization due to hydrotropic properties
- Adsorption
- Micellization
- Detergency (which is a complex combination of several properties)
- Synergistic interactions with other surfactants
In addition, depending on the chemical composition of a particular surfactant, some products may possess important secondary properties including:
- Corrosion inhibition
- Biocidal properties
- Lubricity
- Stability in highly acidic or alkaline media
- Viscosity modification
- Conformance to FDA or BGA regulations for some applications, e.g. direct/indirect food contact
From this list one can immediately see the great number of functions that a surfactant can provide. Also, the difficulty inherent in selecting a surfactant for a particular formulation or set of requirements. As a result, the formulator must know what properties need to be adjusted by the use of the surfactant additive.
Emulsion adhesive films prepared with conventional surfactants can show increased weight by up to 120%. This is due to water uptake, especially at high surfactant concentrations. The water uptake leads to general degradation in both adhesive and cohesive properties of the emulsion film.
In addition to increase in water sensitivity, surfactants may migrate if incompatible, to the adhesive surface. This causes a decrease of tack. Surfactants that are compatible with the adhesive generally act as plasticizers. They may increase tack and peel adhesion and decrease shear resistance. Table below shows the effect of some surfactants on the properties of a pressure sensitive adhesive.
| Surfactant Type |
Amount, phr |
Surface Tension
dynes/cm |
180 Degree Peel Strength
N/m |
Shear Creep Resistance
Hours |
Rolling Ball Tack
cm |
| No surfactant |
0 |
48.7 |
426 |
11.0 |
3.0 |
| Fluorocarbon |
0.02 |
45.9 |
404 |
8.0 |
3.0 |
| Non-ionic |
0.2 |
46.0 |
481 |
6.7 |
3.6 |
| Anionic |
0.2 |
40.0 |
393 |
6.4 |
5.9 |
Effect of Surfactants on the Physical Properties of Pressure Sensitive Adhesives
Surfactant Selection and Formulation Guidelines
Trends Driving Development of New Surfactants
Trends Driving Development of New Surfactants
The recent trends in the development of new surfactants have been mainly related to the issues as discussed below.
Trend 1: Environment Safe Surfactant
Recognition of the environmental issues surrounding higher chain length fluoropolymers has led producers to increase research efforts. The hunt is for the identification of short chain alternatives that provide similar performance characteristics. These efforts will result in the development of additional new fluorochemicals and other surfactant chemistries to ensure the continued growth of the surfactant market.
Growing demand for biosurfactants in developed economies of Europe and North America is expected to create new avenues for industry participants. This is on account of growing consumer awareness and regulatory pressure to reduce reliance on their synthetic counterparts. Governments across these regions have also been promoting the use of biosurfactants which promotes industry participants to increase its production.
Trend 2: Surfactant with Low VOC Level
Most of the efforts in adhesive and coating formulation development have been to reduce volatile organic compound (VOC) emissions. This is done by decreasing the amount of solvent. This has resulted in development and close analysis of surfactants in these formulations in high-volume adhesive systems such as latex emulsions for tape converting which meet strict VOC standards.
Sometimes surfactants used as wetting, flow and leveling agents are fugitive. This implies that they no longer serve a useful function after performing their primary function. Also, they can move about the bulk or surface of any object into which they are formulated. This can have two undesirable consequences:
- The unexpected presence of a surfactant at an inappropriate interface can cause the surface to be much more hydrophobic or hydrophilic than expected for the base formulation.
- The fugitive surfactant can enter the eco-sphere and if toxic create health and safety issues.
Related Read: Formulating Adhesives and Sealants with VOC Restrictions
Trend 3: Surfactant with Low Migration
The optimum path to permanence is through the use of a reactive surfactant. This is accomplished by addition of a curable functional group such as a reactive acrylate that can homo-polymerize (forming an interpenetrating network) or react with other functional groups present in the binder resin.
The surfactant market is extremely diverse and includes many primary product manufacturing industries and segments. Surfactants are used in formulated adhesive products to provide optimum performance. The trends noted above will result in continued development of new products and added value propositions for the adhesive formulator.
Available Surfactants for Adhesives and Sealants
View a wide range of surfactants available today, analyze technical data of each product, get technical assistance or request samples.
References
- Karsa, D. R., “History and Applications of Surfactants” Chapter 1 in Chemistry and Technology of Surfactants, edited by Richard J. Farn., Blackwell Publishing Ltd., 2006.
- D. Myers, Chapter 2 “The Organic Chemistry of Surfactants”, in Surfactant Science and Technology, 3rd ed., John Wiley & Sons, Inc., 2005, p. 33.
- Meng, J., “Novel Applications for Fluorosurfactants in Low-VOC Coatings”, Paint & Coatings Industry, April 2007, pp. 84-88.