What are Tackifiers?
What are Tackifiers?
Tackifiers are important low molecular weight constituents in hot-melt and pressure-sensitive adhesives in terms of their effect on both cost and performance properties. They frequently represent most of the weight percentage (up to 40 percent of total mass) and cost of the adhesive formulation. They are used to increase the tack or "stickiness" of the adhesive.
In practice, formulators use tackifiers to make the correct balance between adhesion and cohesion which is dependent on the end-use application. They do this by lowering the modulus and increasing the glass transition temperature of the final adhesive.
Tackifiers must be considered with regard to:
- The end-properties that the formulator is trying to achieve
- The type of processing that the adhesive will be subject to, and
- The chemical nature of the base polymer
Basic Requirements for Tackifiers
While formulating an elastic adhesive, a rubbery polymer provides the elastic component where as, a low molecular weight tackifying resin constitutes the viscous component. The resin ultimately determines the viscoelastic behavior and the final properties of the adhesive.
Tackifiers tend to have relatively low molecular weight with glass transition and softening temperatures that are above room temperature. They are most often used in an adhesive formulation containing a base polymer that has a Tg below room temperature. Therefore, the tackifier addition raises the Tg of the final adhesive.
Tackifiers operate opposite to plasticizers which lower the Tg of the adhesive formulation.
The tackifying agent must be compatible with the base polymer. With hot-melt adhesives, consideration must also be given to the heat stability of the tackifier in the melt. Tackifiers with unsaturation could potentially gel while the adhesive is in the melt phase. They should also have a relatively low surface tension so as to readily wet the substrate.
Let's understand what happens when tackifiers reduce the modulus and increase the glass transition temperature.
Functions of Tackifiers
Functions of Tackifiers
In adhesive and sealant formulations, tackifiers are used to generate tack and improve specific adhesion (peel strength). They are incorporated into base polymers which mainly lack tackiness and pressure-sensitive properties but provide cohesion. In this way, tackifiers provide the formulator with a tool to balance adhesion and cohesion in line with the end-use requirements.
| Tackifiers |
| Increase Tack and Peel Strength |
Improve Specific Adhesion |
Balance Adhesion and Cohesion |
Promote high temperature adhesive performance |
Decrease Shear or Cohesive Strength |
Tackifiers can be used to adjust the peel, tack, and shear strength of PSA formulations1.
The image below shows the dependence of tack, peel, and shear on the base polymer / tackifier ratio. Note that the properties are highly dependent on the concentration of tackifier in the adhesive formulation and that an optimal concentration must be identified as well as a favorable tackifier chemistry.
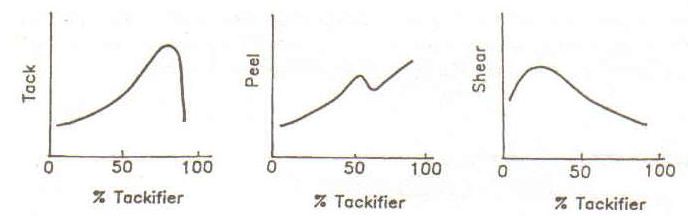 Dependence of Tack, Peel, and Shear on
Resin / Tackifier Ratio1
Dependence of Tack, Peel, and Shear on
Resin / Tackifier Ratio1
Tack is the initial “stickiness” of the adhesive when it contacts the substrate under very slight pressure. Tack is measured in units of force (lb. or N) and is determined by the PSA’s ability to “wet” the substrate. Peel and shear adhesion is about bond breaking – not bond formation.
- Tackifiers have been found to be especially useful in improving adhesion to hard-to-bond substrates such as low surface energy plastics and coated paper.
- They have also been found to promote high temperature adhesive performance in certain adhesive systems.
- Two examples that illustrate the additional benefits of tackifiers are:
- Thermoplastic elastomer systems (e.g., styrene butadiene copolymers) in which
hydrocarbon resins
are used to increase heat resistance, and
- Neoprene contact adhesives, in which phenolic resins are used to improve processing and heat resistance.
In addition to modify tack, adhesion, and cohesion, tackifiers will
affect other properties. These may be advantageous or disadvantageous to the
formulator. Properties such as viscosity, color, color change on aging, thermal
stability, and odor are also dependent on the tackifier chosen and its
concentration. As a result, tackifiers in every formulation need to be tested
against an extended list of criteria as discussed later in this guide.
Types of Tackifiers
Types of Tackifiers
Tackifiers can be derived from natural or petroleum feedstocks and they are divided into three main groups:
- Hydrocarbon resins
- Rosin resins, and
- Terpene resins
Although the natural tackifying resins represent the oldest technology, a recent increase in interest has occurred in these products due to the emphasis on bio-based, green additives. Important characteristics of the various types of tackifying resins are summarized below.
| Main Tackifier Types |
Feedstock |
Characteristics |
Hydrocarbon resins
|
Synthetic
- C5
- C9
- Dicyclopentadiene
- Phenol
- Other
|
- Low molecular weight polymers
- Tg generally above 23°C
- Tg from liquid to 150°C
|
Rosin resins
|
Natural
- Pine trees
- D-Limonene (citrus)
|
- Not polymers but blends of different monomers with low molecular weight
|
Terpene resins
|
- Generally polymeric materials with low molecular weight
- Tg higher than other tackifiers
|
Main Types of Tackifier Resins and Their Feedstocks
#1. Hydrocarbon Resin Tackifiers
Hydrocarbon resin tackifiers are manufactured from petroleum-based feedstock, and therefore, they have the disadvantage of being linked to the price of oil. These resins have a somewhat lower compatibility range with base polymers than tackifiers produced from natural feedstock.
Hydrocarbon resins tackifiers are either aliphatic, aromatic, or mixtures of the two. Hydrocarbon resin tackifiers are polymerized to raise their softening points to a level that is useful in adhesives. They are low molecular weight amorphous synthetic polymers.
Table
below summarizes the most common types of hydrocarbon resin tackifiers. Hydrocarbon resins tackifiers can be hydrogenated for the same advantages that hydrogenation provides rosin resins as was shown in the image above.
|
Types of Hydrocarbon Resin
|
Characteristics
|
|
|
- C5 mainly and some C9
- Wide range of softening points and molecular weight
|
|
|
- C9
- Wide range of softening points and molecular weight
|
|
|
|
|
Pure monomer (aromatic)
|
- Highly aromatic with superior odor, stability, and adhesive performance
|
Hydrocarbon Resins for Tackifying Adhesives
Pure monomer hydrocarbon resins are used to modify the styrene end-blocks of styrene block copolymer. This increases the temperature range and adhesion. Their high polarity has been shown to significantly elevate the performance of EVA-based adhesives.
The major types of hydrocarbon resins are disscussed below.
The feedstocks to produce C5 and C9 hydrocarbon resins are fractions from a naphtha cracker. The feed streams to produce hydrocarbon resins can be divided into two groups — C5 piperylene feedstock and C9 resin oil.
a. C5 Aliphatic Hydrocarbon Resins
C5 piperylene contains various monomers. (See figure below)
The liquid C5 feedstock can be polymerized to a hard resin using a Lewis acid catalyst and carefully selecting temperature and pressure to obtain the desired softening point and molecular weight.
C5 resins are in essence aliphatic materials. They are available in a wide range of softening points and molecular weights.
b. C9 Aromatic Hydrocarbon Resins
C9 resin oil contains various monomers. (See figure below)
A cationic polymerization reaction converts the liquid feed to a hard resin.
C9 resins are aromatic molecules. They are also available in a wide variety of softening points and molecular weight.
C5 and C9 resins can be modified by mixing the two feed streams together in certain ratios. This ratio determines the aliphatic/aromatic balance of the resin, which is essential to formulators. The aliphatic C5 feed can be replaced with a terpene feedstock and modified with styrene to form styrenated terpenes which have excellent color and stability and are very good tackifiers for block copolymers.
There are various physical and chemical parameters that are important to characterize tackifier resins. For hydrocarbon tackifier resins the aromatic/aliphatic balance of the resin is of special interest to adhesive formulators since it largely determines compatibility and ultimately, adhesive performance.
The following criteria to characterize these resins:
- Color, Gardner and Hunter Lab scales
- Softening point, Ring & Ball
- Molecular Weight
- Melt Viscosity
- Thermal stability
- Compatibility, Polarity and Cloud points
c. Dicyclopentadiene Hydrocarbon Resins
Dicyclopentadiene (DCPD) feedstock contains various structures such as those shown in
figure below, but is primarily made up of dicyclopentadiene. The feed stock also contains co-dimers with dienes such as isoprene, butadiene and methylcyclopentadiene. At elevated temperature (170-190°), dicyclopentadiene will crack into cyclopentadiene.
Although the exact structure of DCPD resins is not known, early steps of the thermal polymerization most likely involve the addition of cyclopentadiene to the norbornene olefin followed by continued additions of this type by additional cyclopentadiene to propagate the growing chain.
Dicyclopentadiene is polymerized either thermally or with a catalyst to form relatively dark and unstable resins with a characteristic odor. They are normally used for construction adhesives and inks.
They are more commonly used as a base resin for subsequent hydrogenation to form water white resins with excellent stability and low odor.
d. Hydrogenated Hydrocarbon Resins
Hydrogenation is primarily used to improve color and stability of the resin by removing vulnerable double bonds.
Partial and selective hydrogenation are methods used to produce resins with broad compatibility and good stability.
The most common base resins used for hydrogenation are as follows:
The first hydrogenated hydrocarbon resins were fully hydrogenated, producing aliphatic resins with excellent initial color and stability.
These are:
These resins are ideal for tackifying SIS and SEBS block copolymers.
To produce light colored and stable resins with the correct compatibility to tackify SBS block copolymers and to adjust properties in high styrene SIS block copolymers, two methods were introduced:
- Partial hydrogenation of C9 aromatic resins
- Selective hydrogenation of mixed feedstocks
Hydrogenated C9 Hydrocarbon Resins
C9 resins contain double bonds, which are relatively unstable. A useful way to stabilize these resins is to hydrogenate them. C9 resins have predominantly aromatic ring structures with an overall aromaticity
of around 40%, measured by proton NMR. Resins are hydrogenated in a solution with very specific operating parameters like:
- Temperature
- Pressure
- Hydrogen concentration, and
- Catalyst level
Changing any of these operating parameters will bring a change in the degree of hydrogenation of the final resin. During hydrogenation, the aromatic ring structures gradually lose their aromatic nature and become cycloaliphatic.
When the process is allowed to go to completion, the result is a fully hydrogenated hydrocarbon resin with full aliphatic character. The process can also be adjusted so that partially hydrogenated resins are the end result.
This is very necessary in order to prepare resins for wide use in adhesive formulations and is achieved through sequential, but not total hydrogenation of the rings. This means that partially hydrogenated resins still have some aromatic rings.
The ability to be hydrogenated to varying degrees, resulting in various aliphatic/aromatic balances, gives these resins their unique properties. In theory any degree of hydrogenation can be manufactured. Hercules resins carry a range of hydrogenation levels, varying from 50% to 100%. Example: Regalite™ S5100, Regalite™ 6108
Selective Hydrogenation of Mixed Feedstocks
To obtain resins with comparable compatibility to partially hydrogenated C9 resins, aromatic modification of DCPD (and other cycloaliphatic structures) and C5 resins is necessary.
This is usually achieved by the addition of styrene-based monomers to the aliphatic monomers and subsequently polymerized.
Thereafter selective hydrogenation needs to be performed, in order to reduce unsaturation and improve color of the cycloaliphatic and aliphatic structures without significantly affecting the aromatic content. Example: Regalite™ V6100
#2. Rosin Acids and Esters
Rosin is one of the oldest raw materials for the
adhesives industry, either as such or converted to rosin ester. These resins (rosin acids and rosin esters) are derived from pine tree by-products such as:
- Gum rosin
- It is the oleo resin (pine gum) of the living pine tree.
- The harvesting of the oleo resin is simple, involving only periodic wounding of the tree and collecting of the exudate into cups.
- Wood rosin
- After harvesting pine trees, the stump is allowed to remain in the ground for about ten years so that its bark and sapwood may decay and slough off to leave the heartwood rich in resin.
- Resinous material is extracted from the stump.
- Tall oil rosin
- It is obtained by distillation of crude tall oil (CTO), a by-product of the kraft sulfate
pulping process.
- CTO contains 70-90% acidic material, which is composed
essentially of fatty acid and tall oil rosin. It has the tendency to crystallize and usually contains 200-600 ppm sulfur.
- Highly distilled TOR can produce esters which are competitive with gum and wood rosin derivatives. PAMITE® 79 is an example of tall oil resin.
They provide tack to almost all polymer types but are most often used to tackify natural rubber, ethylene vinyl acetate (both high and low vinyl acetate content), acrylic, styrene butadiene rubber, styrene butadiene copolymers, and polyurethanes.
The main types of rosin acids and esters that are used as tackifiers are given in the table that follows.
Main Types of Rosin Tackifiers
These rosin tackifiers are manufactured in a wide range of molecular weight and softening points, which significantly affects their compatibility with base polymers and their tackifying efficiency. Within these various types, differences in softening point mainly affect compatibility and therefore performance.
Hydrogenation & Esterification of Rosin Resins
Early rosin resin tackifiers had developed a reputation for poor stability caused by unsaturation of the molecule. However, stability can be improved by various methods such as hydrogenation and esterification through rearrangements of the double bonds.
The table below lists other advantages achieved by esterification or hydrogenation of rosin resins.
| Advantages |
| Esterification |
Hydrogenation |
-
Improved oxidative stability relative to rosin acids (improved color
stability)
-
Broad range of softening points depending upon alcohol used
-
Range of compatibility
-
Improved odor
|
- Significantly improved stability
- Light color, less skin sensitivity, less UV absorption
- Can be used in amorphous polyolefin-based adhesives
- Imparts a slight decrease in cohesive strength, especially in block
polymer systems
|
Advantages of Esterification or Hydrogenation of Tackifiers
Chemistry of Rosins
Rosin resins are not polymers, but they are a blend of different molecules. The figure below displays some of structures of rosin molecules.
 Structure of Different Rosin Molecules
Structure of Different Rosin Molecules
Rosin molecules have poor stability caused by unsaturation. Stability can be improved by various methods such as disproportionation, hydrogenation and esterification2.
Rearrangement of the double bonds by disproportionation leads to improved stability.
Another method to improve stability is to hydrogenate the rosin molecules. Example: Staybelite™ Resin-E, Foral™ AX-E
 Disproportionation of Rosin Acids
Disproportionation of Rosin Acids
 Hydrogenation of Rosin Acids
Hydrogenation of Rosin Acids
The carboxylic acid can be converted to an ester using various alcohols. The molecular weight of the alcohol determines the softening point of the subsequent ester.
 Reaction of Rosin Acid with Glycerol
Reaction of Rosin Acid with Glycerol
In the above figure glycerol is used as alcohol. The reaction is an equilibrium reaction, which is driven to near completion. However, there will always be some unreacted acidic and hydroxyl groups.
A typical acid number for a pure rosin acid is around 170. A glycerol ester typically has an acid value below 20. The type of alcohol chosen is
the key to the molecular weight of the rosin ester and its softening point. A typical softening point for glycerol esters is 85°C, and 105°C for pentaerythritol esters. The difference in softening point affects their compatibility and hence adhesive performance.
Rosin resins have a wide span of compatibility with almost all polymers. They are well known for their peel and tack contribution to the adhesive, but generally decrease cohesive strength.
In addition to improved oxidative stability and color, rosin esters impart
excellent adhesion to a wide range of substrates due to their polarity and
compatibility.
#3. Terpene Resin Tackifiers
Another natural tackifier known as the terpenes are sometimes referred to as "universal" tackifiers due to their compatibility with a great many polymers including EVA, amorphous polyolefin, polyethylene, natural rubber, acrylic, styrene butadiene rubber and styrene butadiene copolymers. Terpene resins are especially compatible with polyolefins and the mid-block of styrene-isoprene-styrene resins.
Terpene tackifiers are usually polymerized, and a broad range of softening temperatures is available which depend strongly on molecular weight. The softening point is generally higher than for other tackifiers. The high softening point grades provide excellent heat resistance.
Terpene tackifiers provide improved adhesion for almost all polymer types as well as excellent initial color. These resins can also meet direct food contact requirements. As a result of their properties and natural source, terpene tackifiers have a relatively high use but limited supply. This combination leads to somewhat higher price.
Terpene resins are based on three feed streams:
- Alpha-pinene and beta-pinene are derived primarily from two processes: stump extraction leading to the isolation of steam distilled wood turpentine and the kraft sulfate pulping process leading to the isolation of sulfate turpentine. The individual terpene compounds are isolated by distillation from these two streams.
- d-Limonene is obtained from citrus sources and a similar compound, dipentene, is obtained by distillation from petroleum sources.
 Different Structures of Terpenes
Different Structures of Terpenes
The main difference for the formulator between these resins is that the d-limonene (and dipentene based) resins are not compatible with SBR polymers.
These resins are formed by a cationic polymerization reaction using a Lewis acid catalyst. These resins have excellent initial color and have a broad range of softening points and have been produced for many years.
There are several terpene resins that are used as tackifiers (see table
below).
|
Types of Terpene Resin
|
Characteristics
|
|
Polyterpene
|
- Higher softening point grades combine high heat resistance and excellent adhesive performance.
- Broad FDA clearance for food contact.
|
|
Hydrogenated terpene
|
- Improved color via hydrogenation.
|
|
Styrenated terpene
|
- Mixed aliphatic / aromatic.
- Excellent color and stability.
- Good tackifiers for SBCs and excellent adhesion to polyolefin substrates.
- Softening points in the range of 95°-115°C.
- They have intermediate polarity that provides a broader range of polymer compatibility than unmodified polyterpenes.
- The aliphatic-aromatic structure of these resins provides excellent adhesion to a range of substrates.
- Used in premium applications due to light color and low odor.
|
|
Terpene phenolic
|
- Polar resin with excellent adhesion and broad compatibility with polar, and difficult to bond substrates (e.g., coated and recycled paper).
- Low molecular weight and narrow molecular weight
distribution provide excellent compatibility with polar polymers. As a result, terpene phenolics are used to provide peel strength in EVA and styrene block copolymer hot-melt adhesive systems.
- Very high polarity (due to free phenolic hydroxyl groups).
- High softening point provides (95°-147°C).
- Their excellent hot tack is useful in bookbinding and packaging applications.
|
Terpene Resins for Tackifying Adhesives
#4. Tackifier Resin Dispersion
Incorporation of tackifiers into water-borne adhesive systems has been made
easy with the development of tackifier
resin dispersions. These are usually manufactured by emulsifying the resin as a melt into the aqueous phase.
Once the system has been cooled down, the resin becomes hard again and
remains finely dispersed in the aqueous phase. In certain cases, resins that
are liquid at room temperature are dispersed as well. Tackifier resin dispersion can be mixed into the polymer dispersion easily.
The amount and type of emulsifiers used are the main factors for storage stability, compatibility, and coatability. As a result, a broad range of resin dispersions have been manufactured from rosin and hydrocarbon resin based tackifiers.
Tackifiers Advantages and Disadvantages
Tackifiers Advantages and Disadvantages
Every additive that is added to a formulation either strengthens it or carries some drawbacks. Apprise yourself with the advantages and disadvantages of different types of tackifiers used with adhesive and sealants.
The table below consists of the technical and commercial benefits and limitations of adding a typical tackifier to your adhesive/sealant formulation. Have a look and easily select the tackifier that suits your needs.
Technical
|
| Advantages |
Disadvantages |
| Rosins |
Hydrocarbon Resins |
Terpenes |
Rosins |
Hydrocarbon Resins |
Terpenes |
- Historical experience
- General use
- Range of grades
- Adhesive properties
- Fair converting properties
|
- Adhesive properties
- Fair converting properties
- Good aging resistance
- Some grades are colorless
|
- General use
- Range of grades
- Good aging resistance
- Some grades are colorless
|
- Poor aging resistance
- Yellowing
- Lower adhesion
|
- Restricted usability
- Limited range of grades
|
|
| Commercial |
| |
- Unlimited raw material base
- Unlimited technology
- Lower price
|
- Natural and synthetic grades
|
- Limited raw material base
- Limited technology
- Higher price
|
- Limited raw material base
- Limited technology
- Higher price
|
- Limited raw material base
- Limited technology
- Higher price
|
Selecting Tackifiers for Polymers
Selecting Tackifiers for Polymers
Tackifiers work by reducing the modulus and increasing the glass transition temperature
(Tg) of the resulting adhesive formulations. Both these factors are critically
important to achieve the necessary tack and peel strength. In order to effectively provide these modifications, tackifiers must be compatible or partially compatible with the base polymer.
Reducing the Modulus
In the 1960s, Dahlquist observed a minimum level of compliance that a pressure-sensitive adhesive must have in order for it to exhibit tack.
This can be broadly described as a need for the material to be pliable in order to absorb energy inputs rather than allowing it to propagate through cracks.
The lower the modulus, the easier the PSA adhesive will be to deform, flow, and make good contact with the substrate to which it is bonding. Observations concluded that for pressure sensitivity the adhesive's storage modulus
(G') must be below 3.3 x 105 Pa. This is now known as the Dahlquist criterion.
Tackifiers must have a low to moderate molecular weight (generally between 300 and 2000), which imparts some cohesive strength and prevents the formation of weak boundary layers at the interface - a problem that happens often with low molecular weight plasticizers.
Because tackifiers have lower molecular weight than the base polymer, they dilute the polymeric network and reduce the modulus. A plasticizer by itself could also reduce the modulus, but this is generally an ineffective approach because the Tg of the entire formulation is significantly reduced.
Increasing the Glass Transition Temperature
The glass transition temperature also plays an important role in tack, and tackifiers generally increase the Tg of the adhesive.
At temperatures near the glass transition region, the adhesive is very effective at dissipating energy and providing good adhesive strength. Most base polymers that go into pressure-sensitive adhesives have relatively low Tg – some significantly below room temperature. However, adhesives that are applied and operate near room temperature require a Tg that is nearer to those temperatures. In these cases, tackifiers with Tg from about room temperature to 100°C are used to increase the Tg of the adhesive.
To be an effective pressure-sensitive adhesive, the formulation must have a modulus
below 3.3 x 105 Pa and a Tg near to the application & end-use temperatures.
The image below illustrates the optimum modulus and Tg for various adhesives that are applied and operate at different temperatures.
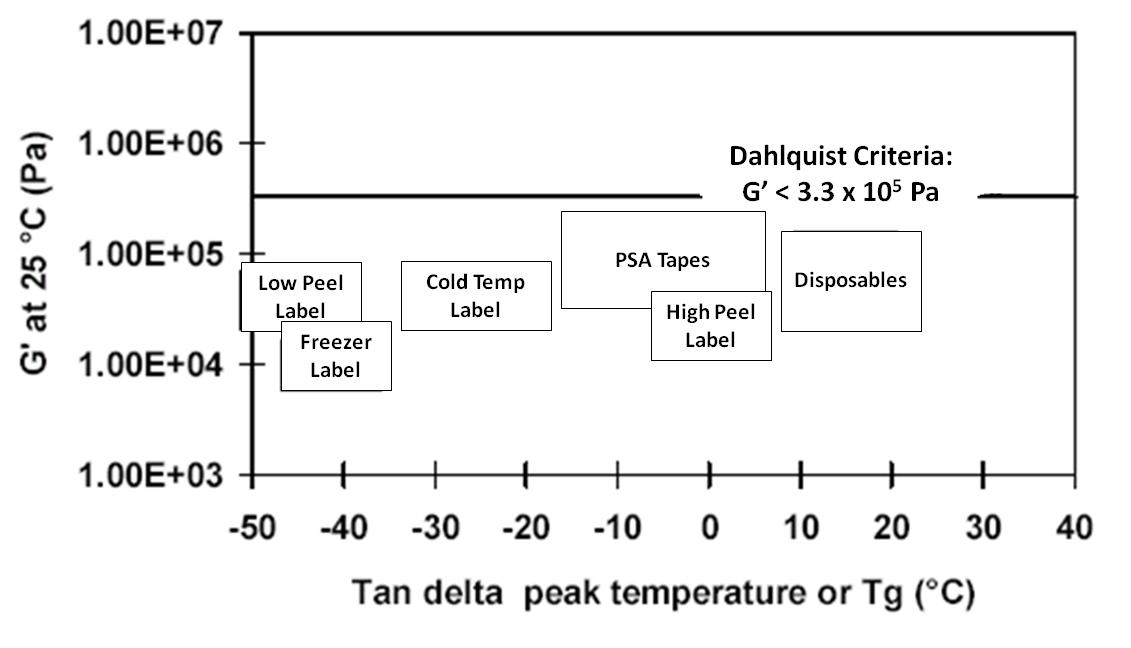 Optimum Storage Modulus and Glass Transition
Temperatures for Different Pressure-sensitive Adhesives
Optimum Storage Modulus and Glass Transition
Temperatures for Different Pressure-sensitive Adhesives
In order to effectively provide these modifications, tackifiers must be compatible or partially compatible with the base polymer.
Compatibility with the Base Polymer
The first and most important criterion in the selection process is the compatibility between the tackifier and the base polymer in the adhesive formulation. Certain classes of tackifiers work best with certain types of polymers. Unless the tackifier is compatible with the base polymer there is no need to extend the selection process. Solubility parameters, molecular weight, and molecular weight distribution determine compatibility.
The higher the compatibility, the more efficient will be the tackification.
If a tackifier is chosen that is incompatible with the base polymer, then it acts like filler. In this case the modulus is increased and there is little change in Tg – circumstances that fail to produce an effective pressure sensitive adhesive.
The tackifying ability of various base polymers strongly differs. For solvent-based adhesives, the versatility range can be listed as follows:
Acrylic > NR >> CR > APO > BR > SIS >> SBS > SBR
For water-borne adhesives the versatility range is given as follows:
Acrylic > NR > CR < BR > EVA > SBR
Hydrocarbon resins can be selected for compatibility based on their cloud points. Cloud point measurements reveal aromatics. Cloud point tests determine polarity and aromaticity by heating the tackifier in two solvents and recording the temperature at which the resin begins to form a separate phase. In such tests, low temperature values indicate high levels of polarity and aromaticity, and tackifiers should be used with polymers demonstrating similar properties.
The tables below provide a general guide to the selection of tackifiers with various common polymers used in hot-melt and pressure-sensitive adhesives. This grouping is based primarily on compatibility and it is a good starting point in the selection of a tackifier.
Care must be taken in the formulation of block copolymer adhesives because they generally have a high temperature thermoplastic end-phase and an elastomeric mid-phase. The tackifier can be directed to either or both phases depending on its compatibility. For example, in styrene block copolymers the formulator may want the polystyrene phase to remain glassy for optimum cohesive properties.
The aliphatic C5 resins, which are more compatible in the non-styrene phase, therefore are more likely to be used. However, in certain applications it may be desirable to increase or decrease the Tg of the end-phase and for this an end-phase compatible tackifier such as aromatic hydrocarbon resin may be used.
Type of Tackifier
|
Acrylic, acrylate |
Asphalt (bitumen) |
Polyamides |
Polyesters |
Amorphous (APO) |
Polyethylene (PE),
Polypropylene (PP) |
Metallocene (MePE) |
Hydrocarbon Resins
|
√
|
|
|
|
√
|
√
|
|
Pure Monomer
|
√
|
|
|
√
|
√
|
|
|
|
Hydrogenated Hydrocarbon Resin
|
|
√
|
|
|
√
|
√
|
√
|
Resin Dispersions
|
√
|
|
|
|
|
|
|
Rosin Esters
|
√
|
√
|
√
|
√
|
√
|
√
|
|
Rosins
|
√
|
√
|
√
|
√
|
√
|
√
|
|
Terpenes
|
√
|
|
|
√
|
|
√
|
|
Select Tackifiers for Rubbers
|
Base
Polymer |
Hydrocarbon Resins |
Pure Monomer |
Hydrogenated Hydrocarbon Resin |
Resin Dispersions |
Rosin Esters |
Rosins |
Terpenes |
| Chlorinated |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
| Natural
|
|
|
|
√ |
|
|
|
| Butyl
|
√ |
|
√ |
|
√ |
√ |
|
| Polysulfide
|
√ |
|
|
|
|
|
|
| PU, thermoplastic |
√ |
√ |
|
√ |
√ |
|
|
| PU, thermoset
|
|
|
|
|
√ |
√ |
|
| SBCs (midblock) |
√ |
√ |
√ |
|
√ |
√ |
√ |
| SBCs (endblock) |
√ |
√ |
√ |
|
|
|
|
| Styrene rubber |
√ |
√ |
√ |
√ |
√ |
√ |
|
| Neoprene |
|
|
√ |
√ |
√ |
√ |
√ |
Select Tackifiers for Vinyl Polymers
Type of Tackifier
|
Ethylene copolymers (VAE, EVC, etc.) |
Ethylene co- terpolymers solid (EVA, EMA, etc.)
|
Polyvinyl acetate (PVAC) |
Polyvinyl butyral (PVB)
|
Polyvinyl chloride (PVC) |
Polyvinyl pyrrolidone (PVP) |
Hydrocarbon Resins
|
√
|
√
|
√ |
|
√
|
|
Pure Monomer
|
√
|
√
|
|
|
|
|
|
Hydrogenated Hydrocarbon Resin
|
|
√
|
|
|
|
|
Resin Dispersions
|
√
|
|
|
|
|
|
Rosin Esters
|
√
|
|
|
|
|
√
|
Rosins
|
√
|
√
|
√
|
√
|
√
|
√
|
Terpenes
|
√
|
√
|
√
|
|
√
|
|
Solubility
One can either employ solubility parameters or other parameters to estimate the compatibility of a tackifier with a certain polymer. However, usually trial and error testing is required. Materials that possess similar solubility parameters are generally assumed to be compatible with each other.
The image below illustrates solubility parameters for various base polymers and tackifiers.
Solubility parameters for base polymers and tackifiers
IF a polymer and the tackifier are compatible a single Tg is observed but shifted to a higher temperature due to the higher Tg tackifier resin. In case of incompatibility, there will be two different Tgs, one polymeric phase and one resin phase.
Softening Point
In systems having good tackifier / polymer compatibility, softening point is an important parameter relative to the selection of a tackifier.
In general, soft resins impart aggressive tack, while harder resins help retain good cohesive strength and creep resistance.
For the same tackifier family, a low softening point resin will have better compatibility in a specific polymer than a higher softening point.
A higher softening point resin is less miscible with the base polymer. Softening temperatures of commercial tackifiers range between room temperature to about 115°C, and all types of tackifiers can be manufactured within this softening point range.
» Learn how tackifiers work by reducing the modulus!
Some Tackifying Resins with Different Glass Transition Temperatures and Softening Points3
Other Criteria in Selecting Tackifiers
Other Criteria in Selecting Tackifiers
Once compatibility, modulus, and Tg have been determined, other criteria can be considered. These will depend on the requirements of the adhesive and its application. Examples of important properties to consider are provided below.
- Concentration (Resin Level required)
- Stability
- Compounding and application properties
- Specific adhesion to the substrate in question
- Odor
- Color (initial and on aging)
- Other physical and chemical properties
- Efficiency and cost
Resin Level Required
It is difficult to predict the resin level required to achieve a certain tack or peel value because of the dependence on the nature of the base polymer and the tackifier. The type of substrate that is being bonded should be taken into account as well.
A level of up to 30-40% by weight of tackifying resin is used in formulating many acrylic-PSAs for bonding to polyolefin film.
A modulus increase will usually begin in the range of 40-60% resin loading. However, more than 20% tackifier resin generally results in lowering of cohesive strength. For natural rubber-based PSAs tackified with hydrocarbon resins, maximum tack is obtained at about 50-65% tackifying resin, and optimum peel strength is obtained at a concentration of about 50% by weight.
Stability
After determining the compatibility, stability is the next most
important tackifier characteristic to consider. The stability of the plasticizer will depend on the compounding and application processes that the adhesive will need to go through as well as the environment (heat, oxygen, chemicals, etc.) once the adhesive is placed in service.
Additives may be used in the adhesive formulation to prevent stability problems associated with the tackifiers. These include antioxidants and other stabilizers.
With hot-melt adhesives, consideration must also be given to the
heat stability of the tackifier in the melt. Tackifiers with unsaturation could potentially gel while the adhesive is in the melt phase. Heat stability can often be approximated by the:
- Chemical composition (aromatic > aliphatic) and
- Glass transition or softening temperature of the tackifier
When using dispersions of tackifying resins in water-borne adhesives, stability testing will ensure that there are no undesirable reactions between the emulsifier systems in the tackifier and base polymer dispersions.
Compounding and Application Properties
Tackifiers are sold either as 100% non-volatile forms (mainly solids and some liquids), as solution cutbacks, or as water-borne resin dispersions. The choice of form will depend on the processes required to formulate the adhesive and specific logistic or regulatory requirements.
High viscosity tackifying resins are usually pre-warmed for easier handling.
Resins that have a high melting point should be added early in the mixing cycle
in order to guarantee melting and sufficient dispersion. Soft resins can be added together with filler and other additives to make use of their wetting and dispersing properties, and relatively late addition can be useful for building maximum tack.
The type of tackifier used can significantly affect the viscosity of the adhesive system. For a given temperature, the viscosity can vary up to 5 times or more depending on the type of plasticizer used. Image below illustrates the variety of viscosities that can be achieved by a given SIS polymer system depending on the choice of tackifier. As a result, tackifiers are sometimes used to fine-tune viscosity requirements.
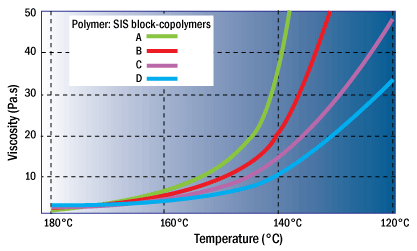 Influence of tackifier choice on the viscosity of an SIS hot-melt adhesive system
Influence of tackifier choice on the viscosity of an SIS hot-melt adhesive system
Tackifiers Selection for Pressure Sensitive Adhesives
Tackifiers Selection Checklist