UV Light and Material Degradation
UV Light and Material Degradation
Ultraviolet radiation (UV) from sunlight is an important component in outdoor degradation. Damage caused by the action of light plays a role that should not be underestimated in many different areas of life. The destructive effects of light can lead to irreversible changes in organic materials, such as adhesives.
Although adhesives are generally located between two substrates that are being bonded together, damage can still occur in such cases at the edges of the adhesive bond or if the substrates are permeable to light which may produce discoloration or the loss of adhesive properties.
In substrates, UV radiation can change the mode of stress due to changes in the substrate’s modulus, introducing weak boundary layers at the interface, or increasing stress due to shrinkage of the substrate.
Further, UV degradation is not usually a problem if both adherends are opaque; however, it can be critically important when bonding glass, UV transparent plastics, and in designing joint where free edges and fillets of adhesive might be exposed to sunlight.
UV resistance is a major issue in sealants, potting and encapsulating compounds, packaging compounds, labels and tapes, etc. when these materials must be used outdoors. It is also important in thermoplastic formulations such as
hot-melts because of their propensity to degrade by UV attack.
Let's understand the mechanism of photodegradation, its effects and prevention in detail...
Mechanism of Photodegradation
Mechanism of Photodegradation
Once the adhesive or sealant is exposed to shear stress, heat, light, air, water, radiation or mechanical loading, chemical reactions start in the polymer backbone. These reactions have the net result of changing the chemical composition and the molecular weight of the polymer.
These reactions, in turn, lead to a change in the physical and optical properties of the polymer and adhesive resulting in embrittlement, discoloration and overall reduction in the physical properties of the material.
The amount of UV absorbed by an adhesive or sealant, the degree of degradation, and the nature of the chemical reactions that occur during degradation all depend on the molecular structure of the base polymer and on substances present in the formulation.
Photo-oxidation of many polymers is often modeled on a scheme originally developed for natural rubber:
The important aspect of this scheme is that once oxidation starts, it sets off a circular chain reaction that accelerates degradation unless stabilizers are used to interrupt the oxidation cycle.
Effects of Photodegradation
Exposure to sunlight and some artificial lights can have adverse effects on the useful life of adhesives or sealants. UV radiation can break down the chemical bonds in a polymer. This process is called photodegradation and ultimately causes cracking, chalking, color changes and the loss of physical properties.
Photodegradation, once started, essentially follows the same scheme as shown above. Since photodegradation generally involves sunlight, thermal oxidation takes place in parallel with photo-oxidation. Photo-oxidation can be so pronounced with adhesives too that they would be destroyed completely within a few days or weeks if they were used directly exposed to light without any protection.
How to Prevent UV Degradation?
How to Prevent UV Degradation?
There are several approaches that can be used to prevent the photo-oxidation of an adhesive or sealant, such as:
- Inhibit the UV light from attacking the adhesive/sealant. This can be done possibly by using the substrate, itself, or the geographic location of the joint as a barrier to UV.
- Incorporation of additives into the formulation that will stabilize it against the UV deterioration processes.
- Alloying or blending two different polymers (one with better UV stability).
- A combination of all approaches above.
These approaches are not always practical or even possible. Also, the impact of antioxidants, that can help to guarantee stability to a varying extent with a given light stabilizer system, should not be underestimated as a factor in optimization of the light stabilization of adhesives.
Generally, the edges of the joint remain exposed and may discolor and begin to peel from the substrates. Sealant applications, of course, have a significant amount of surface area exposed to the sunlight; thus, discoloration and physical changes caused by UV deterioration are of special concern.
A better approach at minimizing the effects of UV is through proper selection of ingredients and formulation of the adhesive or sealant, itself. The burden of UV protection is lifted from the substrate material. In especially serious cases, both the substrate and the adhesive/sealant can be formulated for UV protection.
Types of UV Stabilizers
Types of UV Stabilizers
UV stabilizers are used to solve the degradation problems associated with exposure to sunlight. UV stabilizers can be categorized as:
UV Screeners
UV screeners are actually pigments, they render the polymer translucent or opaque. In this way they absorb or reflect UV radiation and protect the polymer.
- Carbon black can be used in concentrations of only 1-2%.
- High loadings of titanium dioxide, zinc oxide, and other pigments are also effective.
UV screeners are used more as an additive to the substrate rather than as an adhesive additive. However, UV screeners are used in sealant formulations where high degrees of fillers can be tolerated.
Ultraviolet Absorbers
A very simple way to protect adhesives against UV light is to prevent UV absorption, i.e. reducing the amount of light absorbed by chromophores. This can be achieved by incorporating UV absorbers in the adhesives, which function by preferentially absorbing harmful ultraviolet radiation and dissipating it as thermal energy.
Such stabilizers function according to the Beer Lambert law, which specifies that the amount of UV radiation absorbed is a
function of both sample thickness and stabilizer concentration.
In practice, high concentrations of absorbers and sufficient thickness of the polymer are required before enough absorption takes place to effectively retard photodegradation. UV absorbers that are commonly used are benzophenones, benzotriazoles, aryl esters, oxanilides, acrylic esters, and formamidine.
| UV Absorber |
Characteristics |
| Benzophenones |
Moderated absorption in the 390-230 nm range. High temperature versions available for high temperature processing. Possible objectionable color. |
| Benzotriazoles |
Intense UV absorption at 390-280 nm. Good initial color and color stability. |
| Aryl esters |
Undergoes light induced rearrangement to form hydroxy-benzophenone derivatives. FDA acceptance. |
| Oxanilides |
Provides absorption form 320-280 nm. Very low color, low volatility, suitable for high temperature processing. |
| Acrylic esters |
Strong absorption from 320-290 nm. Usually less effective than others. Good initial color and stability on aging. |
| Formamidine |
Broad absorption range. |
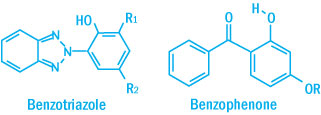 Typical Benzophenone and Benzotriazoles Structure
Typical Benzophenone and Benzotriazoles Structure
Mechanism of Action of UV Absorbers
The different substituents in the benzotriazole group affect various properties, such as polarity, volatility, compatibility, physical condition and maximum absorption levels. Typical UV absorption spectra of benzotriazoles is:
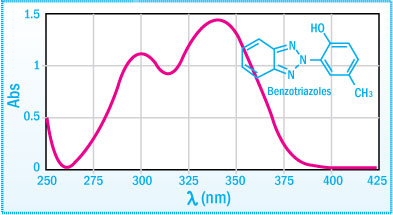 UVA spectra of Benzotriazoles
UVA spectra of Benzotriazoles
The absorption curves show that the requirements are met i.e. strong absorption in the UV range between 295 and 400 nm and a large reduction in absorption in the visible range above 400 nm. The typical protection mechanism of benzotriazoles and benzophenones are illustrated in the schemes below.
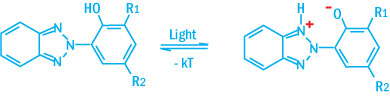 Protection Mechanism of Benzotriazoles
Protection Mechanism of Benzotriazoles
UV absorption causes the electron density to move from the phenolic oxygen to the nitrogen atom. The nitrogen becomes more alkaline than the oxygen as a result and a proton transfer occurs. This mesomeric form represents an excited state, which stabilizes as a result of a radiationless transition to the ground state.
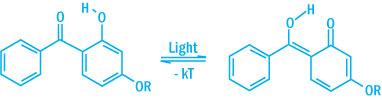 Protection Mechanism of Benzophenones
Protection Mechanism of Benzophenones
UV Quenchers
UV quenchers also inhibit initiation although at a later stage than absorbers. They operate much like UV absorbers. However, unlike absorbers they are effective in thin sections. Quenchers are generally organic nickel compounds such as nickel salts. They are typically used in polyolefins. They impart an initial color to the polymer.
Scavengers and Decomposers (HALS)
Scavengers and decomposers operate later in the photodegradation sequence by inhibiting propagation rather than initiation. They operate through a combination of scavenging and terminating free radicals and decomposing hydroperoxides to non-radical species. The use of hindered amine light stabilizers (HALS) is extremely effective in adhesive formulations.
Hindered amine light stabilizers (abbreviated as HALS) are derivatives of 2,2,6,6-tetramethyl piperidine. They are extremely efficient stabilizers against light-induced degradation of most polymers, such as polyolefins, styrenics, polyurethanes, polycarbonate, and cellulosics.
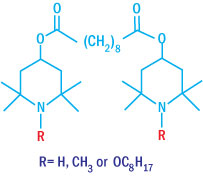 Chemical Structures of Low MW Hindered Amine Light Stabilizer
Chemical Structures of Low MW Hindered Amine Light Stabilizer
Mechanism of Action of Hindered Amine Light Stabilizers
HALS do not absorb UV radiation, but act to inhibit degradation of the polymer. They slow down the photochemically initiated degradation reactions, to some extent in a similar way to antioxidants.
One advantage of the hindered amine light stabilizers is that no specific layer thickness or concentration limit needs to be reached to guarantee good results. Significant levels of stabilization are achieved at relatively low concentrations. HALS' high efficiency and longevity are due to a cyclic process wherein the HALS are regenerated rather than consumed during the stabilization process.
The mechanism of hindered amines stabilizers against thermo-oxidation appears to be complex. Because of the regenerative nature of this process, as well as the typically high molecular weights of the stabilizers, hindered amine stabilizers can provide extreme long-term thermal and light stability.
The hindered amine light stabilizers mechanism involves several different reactions that are responsible for the stabilizing effect they have on photo-oxidation. Proof has been found of the radical entrapment qualities of carbon radicals, the reaction of HALS metabolites with peroxy radicals and the decomposition of hydroperoxides as shown below.
- An acidified hindered amine cannot easily enter the free radical scavenging cycle.
- N-H and N-R hindered Amine Stabilizers fit most needs regarding light stability but can be alkaline.
- N-OR type enter the UV stabilization cycle quickly and are much less alkaline than N-H or N-R type of HAS.
At the end, what tends to be important for the choice of the light stabilization system is the chemical nature of the polymers used and tackifier resins, that will be discussed later. Interaction has also been observed with fillers and pigments. The appropriate use of a light stabilization system can inhibit UV induced reactions that negatively affect adhesives, increasing the service life of the adhesive and improving the total appearance of the adhesive or sealant.
Benefits of Light Stabilizers in Adhesives
Benefits of Light Stabilizers in Adhesives
As mentioned above, UV-induced degradation can occur at the edge of the adhesive bond or if the substrates are more or less transparent to light. If unprotected, the resulting damage caused by photo-oxidation can lead to discoloration or even worse, the loss of adhesive properties of the adhesive or sealant.
Main benefits light stabilizers impart to adhesives are:
Color Stability in Adhesives
The effect of UV induced degradation of an adhesive or sealant often results in several different modes of damage. A critical mode is discoloration. This usually manifests itself by a yellowing or darkening of the adhesive or sealant. Color development, which may occur at widely differing amounts depending on the polymer, is also often accompanied by a loss in adhesive properties.
The following pictures illustrate the effect of photodegradation on a polyurethane
hot-melt adhesive. Both samples were placed in a weatherometer for 12 hours at 65°C. After only 12 hours, the sample without the use of a UV absorber was already yellow.
 |
 |
| Blank |
0.5% UVA |
For adhesives and sealants that may be used outdoors or in applications susceptible to UV attack, protection from discoloration is essential in maintaining the appearance and integrity of the bond or assembled part.
Retention of Adhesives Properties
Although the most visible result of photodegradation is a change in the appearance of a material, mechanical and physical properties are also altered as a result of photodegradation. In adhesives and sealants, these changes are observed and experienced by changes in adhesive performance.
UV light induced oxidation and subsequent degradation leads to loss of adhesive properties, such as tack, adhesive or cohesive strength. The appropriate use of a light stabilization system can inhibit UV induced reactions that negatively affect the adhesive and furthermore, increase the service life of the adhesive. A combination of a UVA and HALS can also be used for optimized effects.
Having learnt about different type of UV stabilizers available and benefits, now explore how light stabilizers can help to improve different formulations from UV light induced degradation:
Light Stabilizers in Sealants
Light Stabilizers in Sealants
The number of applications for sealants and caulks in construction, industrial and consumer markets is continuously growing. Sealants are required to seal and adhere to a wide range of substrates and over an extensive and varied range of temperatures and environmental stresses.
As more and more sealants are being developed and used to satisfy the demanding needs of the industry, increased performance requirements are essential. The sealant must often demonstrate adhesion and movement capabilities, while resisting the deteriorating effects of heat, light and environmental pollutants.
Let’s focus on how light stabilizers can improve the resistance of different sealant formulations to UV light induced degradation:
Polyurethane Sealants
Polyurethane (PUR) sealants and adhesives are used in wide range of applications because of their excellent:
- Toughness
- Abrasion resistance
- Elongation
- Recovery
Despite all these benefits, PUR sealants have some limitations, one of which is sensitivity to UV radiation.
Aromatic polyisocyanates such as toluene diisocyanate (TDI) and diphenylmethane isocyanate (MDI) are commonly used in urethane sealant production. Although polyurethanes derived from TDI tend to be slightly more stable than MDI, all aromatic polyisocyanates contribute to the yellowing of a urethane on exposure to light.
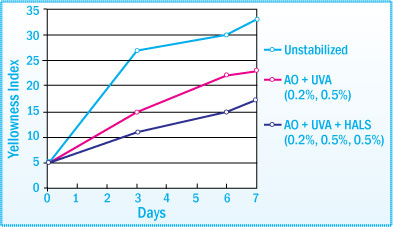 Stabilization of PUR Sealant Discoloration after Light Exposure (50°C)
Stabilization of PUR Sealant Discoloration after Light Exposure (50°C)
To prevent light induced degradation of a polyurethane sealant, which can result in discoloration as well as a loss of adhesive properties, an antioxidant and light stabilizer system is recommended.
Silyl-terminated Polyethers-based Sealants
Silyl-terminated polyethers-based sealants have achieved reasonable market penetration in the last few years and are widely used in construction and industrial applications. Their main advantages are that they exhibit many of the benefits that are offered by silicone and polyurethane sealants, being very strong, but elastic at the same time.
As with many materials, silyl-terminated polyether sealants must be protected against both thermal and UV degradation to ensure optimum product performance. For this reason, standard one component formulations based on these products generally contain both an antioxidant and light stabilizer.
Standard one component formulation
| Component |
Parts (wt) |
| Silyl-terminated Polyethers |
100 |
| Plasticizer |
55 |
| Calcium Carbonate |
120 |
| Titanium Dioxide |
20 |
| Thixotropic Agent |
2 |
| Antioxidant |
1 |
| UV Absorber / Hindered Amine |
2 |
| Dehydrating
Agent |
2 |
| Adhesion Promoter |
3 |
| Hardening Catalyst |
2 |
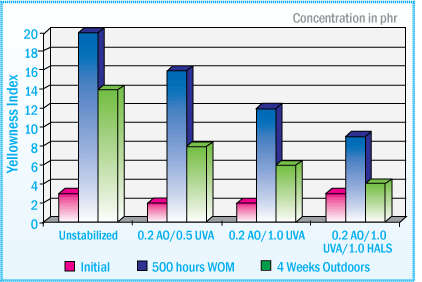
As seen in the graph below, tensile strength performance of a silyl-terminated polyether sealant is enhanced when stabilized with 0.3%-0.45% of a light stabilizer system. The sealant that was not stabilized, lost all its strength after 2500 hours in a weatherometer.
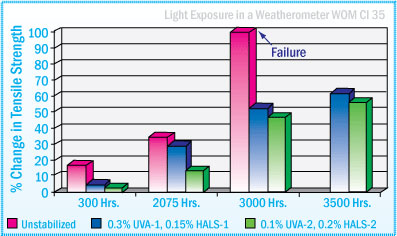 Silyl-terminated Polyethers Sealant - % Change in Tensile Strength After Exposure in WOM
Silyl-terminated Polyethers Sealant - % Change in Tensile Strength After Exposure in WOM
Sealants Based on SEBS / SBC
Styrene butadiene block copolymers (SBC) are widely used in adhesives
& sealants because they offer good cohesive strength, and can be formulated & applied without the use of solvents or crosslinking agents.
Because SBCs with hydrogenated polybutadiene (SEBS) or hydrogenated polyisoprene (SEPS) midblocks are more difficult to crosslink than their non-hydrogenated equivalents, sealants based on saturated polymers tend to have better resistance to oxidative and UV degradation. Nevertheless, UV stabilization is still necessary in order to maintain the appearance and performance of a SEBS sealant.
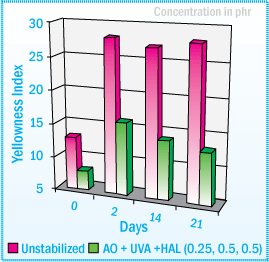
Looking at a SEBS sealant containing a hydrogenated tackifier, the unstabilized sealant discolored and exhibited significant surface crazing after 2000 hours exposure in a Xenon Arc WOM.
Benzotriazole UVAs provided the best color stability and a 0.5% combination of UVA and HALS (1:1) offered the best protection against surface crazing. Even after 2000 hours exposure in a Xenon Arc WOM, the sealant using a combination of the UVA and HALS showed no signs of surface crazing.
The figure below represents the gardner color after exposure in a Xenon Arc WOM:
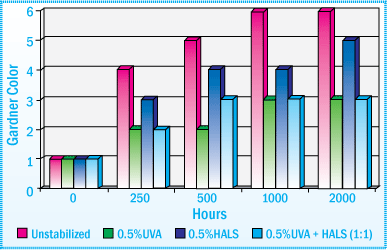 Gardner Color After Exposure in a Xenon Arc WOM
Gardner Color After Exposure in a Xenon Arc WOM
Silylated Polyurethane Sealants
Silylated polyurethane sealants are a relatively new addition to the sealant family. They have achieved interest in the market because of the competitive properties that they offer compared to other conventional sealing products. For instance, with the appropriate use of light stabilizers, silylated polyurethanes offer:
- Fast ambient moisture curing
- Good adhesion to various substrates
- Improved weatherability
- Light resistance
Although formulations will vary to a large extent, standard silylated polyurethane sealants may be characterized by the formulation below.
| Component |
Parts (wt) |
| Silylated polyurethane prepolymer |
100 |
| Plasticizer |
40 |
| Fillers |
100 |
| Titanium Dioxide |
2 |
| Thixotropic Agent |
6 |
| UV Stabilizers (UVA / HALS) |
2 |
| Adhesion Promoter |
2.5 |
| Catalyst |
0.2 |
Light stabilizers are generally used at levels of one part per 100 resins. Because of the absence of residual isocyanate, the effectiveness of the stabilizers is enhanced. A combination of a UVA and HALS was found to offer outstanding protection in a weathering analysis. Using benzotriazole, the color and the surface structure of the silylated polyurethane sealant remained virtually unaffected after almost a year exposure in a weatherometer. In addition, the sealant still exhibited good flexibility.
Light Stabilizers for Hot melt Adhesives
Light Stabilizers for Hot melt Adhesives
EVA-based hot-melt adhesives are widely used in packaging, bookbinding or wood assembly. In some cases, long term performances are needed, and these adhesives have to be protected against degradation linked to light exposure.
SIS-, SBS-, and SEBS-based hot-melt adhesives have enjoyed strong growth in many markets. In numerous applications,
hot-melt adhesives are exposed to either direct or indirect UV light, which can lead to the induced oxidation and subsequent degradation of the adhesive.
Let’s focus on how light stabilizers can improve the resistance of various hot-melt adhesives from UV light induced degradation. The results will illustrate how the appropriate use of a light stabilization system can not only extend the service life of the adhesive, but also improve the total appearance of the adhesive. Factors, which are vital in so many applications, including those for tapes, labels and packaging.
EVA-based Hot-melt Adhesives
Ethylene-vinyl acetates (EVA) are popular because of their superior adhesion to most substrates as well as their ease of formulation. They are used in a wide variety of applications including:
- Packaging (i.e. case and carton sealing)
- Bookbinding
- Non-woven
- Furniture assembly
EVA hot-melt adhesives are generally formulated with large quantities of tackifiers or extenders, such as petroleum and synthetic waxes. A typical EVA-based HMA will consist of the polymer, tackifying resin and petroleum wax in varying concentrations depending on the performance requirements of the adhesive. Antioxidants, light stabilizers, fillers and plasticizers are commonly used to enhance certain properties. Typical composition proportions are:
- EVA Copolymer - 30-40%
- Tackifier - 30-40%
- Wax - 20-30%
- Antioxidant / UVA or HALS - 0.5-1%
Although effectively stabilized raw materials are critical to produce EVA
hot-melts, this does not mean that the basic stabilization will be sufficient to overcome both oxidative and photo-degradation occurring during compounding and end use of the adhesive. If low color and good age stability is regarded as important features of the EVA
hot-melt adhesive, the use of either a UVA or HALS, or combination of both, with an antioxidant is essential.
SIS-based hot-melt Adhesives
Because SIS and SBS are sensitive to light, they need to be stabilized in order to protect them from UV induced reactions. For discoloration and retention of adhesive properties, exceptional results can be achieved with both HALS and UVAs in an SIS-HMA.
Determining which light stabilizer or stabilizer system to use will vary depending on the adhesive formulation, as there can be distinct differences in the results achieved by either a HALS or UVA.
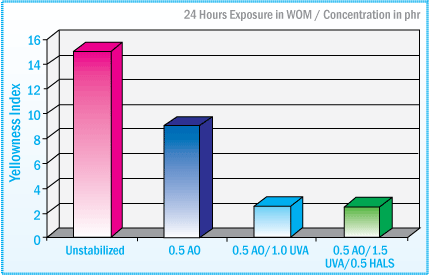
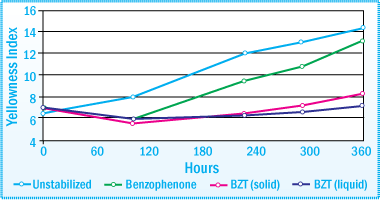 Discoloration of SIS-HMA after Exposure in a Weatherometer Evaluation of UVA Stabilizers at 1% Concentration
Discoloration of SIS-HMA after Exposure in a Weatherometer Evaluation of UVA Stabilizers at 1% Concentration
The image above illustrates the effectiveness of benzotriazole (BZT) UVAs in significantly reducing the amount of discoloration of an SIS-HMA after exposure for 360 hours in a weatherometer. N-methyl HALS are also extremely effective compounds for inhibiting the discoloration of the SIS-HMA.
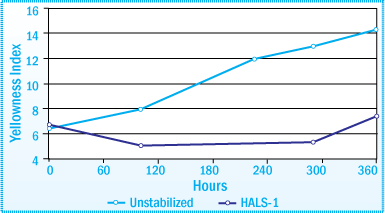 Discoloration of SIS-HMA after Exposure in a Weatherometer Evaluation of HALS at 1% Concentration
Discoloration of SIS-HMA after Exposure in a Weatherometer Evaluation of HALS at 1% Concentration
The appropriate concentration of light stabilizer that is used should be determined by the requirements, keeping in mind that reducing the amount of stabilizer will impact the final results.
In addition, synergistic effects can sometimes be achieved by using a combination of a UVA and HALS.
For retention of initial tack properties, both UVAs and HALS are extremely effective. Even at lower concentrations of 0.3%, UVAs are still able to maintain the performance of the SIS-HMA after 14 weeks of exposure to daylight. The discoloration of the
hot-melt adhesive, however, is more evident with lower concentrations of UVA (images below).
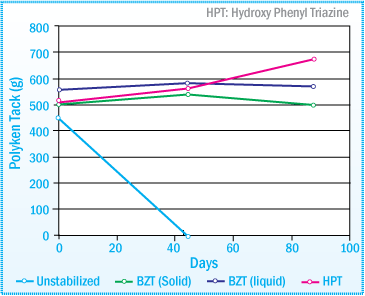 Polyken Tack of SIS-HMA after Daylight Exposure: 1% Concentration of UVA
Polyken Tack of SIS-HMA after Daylight Exposure: 1% Concentration of UVA
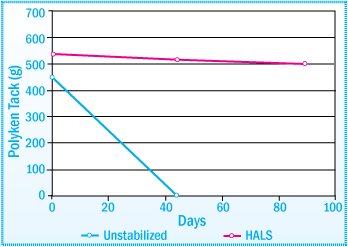 Polyken of SIS-HMA after Daylight Exposure 1% Concentration of HALS
Polyken of SIS-HMA after Daylight Exposure 1% Concentration of HALS
SBS-based Hot-melt Pressure Sensitive Adhesives
SBS and SIS-based adhesives typically exhibit comparable light stability. As with SIS-based HMAs, the unsaturated SBS hot-melt adhesive is not as light stable as a SEBS-based adhesives, as the SBS copolymers have the tendency to crosslink under oxidative conditions.
As a result, antioxidants are used in SBS hot-melt adhesive formulations to protect it against thermally induced oxidation that can prematurely age the adhesive. To protect the adhesive from light induced oxidation, a light stabilizer should also be used. This factor is even more crucial in applications where the
hot-melt adhesive is expected to receive exposure to direct or indirect UV light.
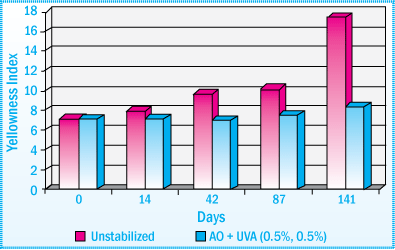 Light Stability of SBS HMA: Exposure to Daylight
Light Stability of SBS HMA: Exposure to Daylight
The images illustrate how a SBS HMA discolors and loses its tack properties when exposed to daylight. Using a UV absorber in conjunction with an antioxidant can help the adhesive retain its color and adhesive properties. Typically, the choice of the antioxidant will not influence the performance of a UV absorber.
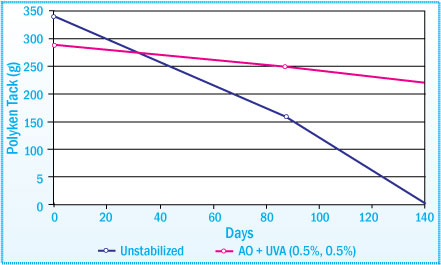 Polyken Tack of SBS-HMA After Daylight Exposure
Polyken Tack of SBS-HMA After Daylight Exposure
SEBS-based Hot-melt Pressure Sensitive Adhesives
Adhesives that contain exclusively saturated compounds are inherently more stable to light than adhesives based on unsaturated compounds. As a result, SEBS-based
hot-melts are somewhat more resistant to light induced degradation compared to SIS-based
hot-melts. Although the effects may take more time to develop, SEBS-HMAs are still susceptible to the effects of degradation: discoloration and the loss of adhesive properties.
UVAs and HALS are both useful in preventing the discoloration of a SEBS-HMA. As seen in the graph below, HALS are somewhat more effective here than UVAs. In terms of the retention of tack properties, the UVAs were for the most part more effective than the HALS. As a result, the combined use of a UVA and HALS can provide synergistic effects.
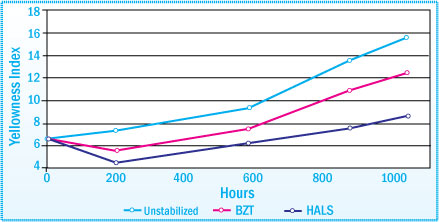 Discoloration of SEBS-PSA after Exposure in a Weatherometer:
Evaluation of Light Stabilizers at 1% Concentration
Discoloration of SEBS-PSA after Exposure in a Weatherometer:
Evaluation of Light Stabilizers at 1% Concentration
SIS-based hot-melt PSA with Hydrogenated Rosin Ester
With the expanding use of PSA tapes and labels in often demanding applications, the retention of adhesive properties has become even more crucial. The adhesive should demonstrate minimal change in adhesion properties and minimum discoloration under ultraviolet light aging conditions. That is the reason proper selection of a light stabilizer is so important.
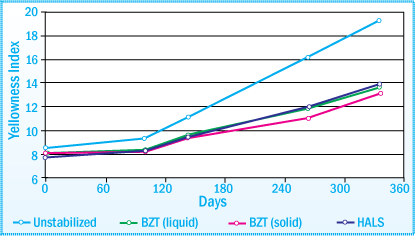 Discoloration of SIS-PSA after Exposure in a Wetherometer: Evaluation of UVA and HALS at 1% Concentration
Discoloration of SIS-PSA after Exposure in a Wetherometer: Evaluation of UVA and HALS at 1% Concentration
The photostability of a SIS combined with a hydrogenated rosin ester is much lower than a SIS plus a hydrogenated hydrocarbon resin adhesive.
As a result, when hydrogenated rosin esters are used, it is even more important that the SIS
hot-melt be protected with either a UVA or HALS in order to:
- Minimize discoloration
- Improve the tack properties
Although the performance between a UVA and HALS in terms of discoloration is comparable, for the most part, UVAs are more effective than HALS in the retention of tack properties.
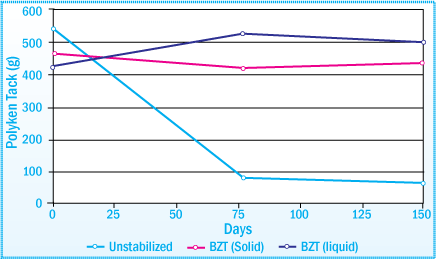 Polyken Tack of SIS-PSA after Daylight Exposure: 1% Concentration Light Stabilizer
Polyken Tack of SIS-PSA after Daylight Exposure: 1% Concentration Light Stabilizer
Light Stabilizers for PUR Reactive Hot melt Adhesives
Light Stabilizers for PUR Reactive Hot melt Adhesives
Polyurethane (PUR) reactive hot-melt adhesives are based on a combination of PUR and hot-melt technology.
- The hot-melt is a 100 percent solid, one-component urethane prepolymer.
- It is fast setting, like a standard hot-melt, but then reacts with atmospheric or substrate moisture to cross-link or chain extend, forming a new polyurethane polymer.
- This combination results in a hot-melt with enhanced performance.
Unlike conventional thermoplastic adhesives, where remelt when heated, PUR
hot-melts cure to a thermoset material, retaining their structural integrity once cured. In addition, the cured adhesive:
- Has excellent temperature and environmental resistance, generally withstanding exposure to temperatures from -30°C to +150°C
- Maintains strong bonds between similar and dissimilar substrates
|
|
|
|
Blank
|
0.5% UVA
|
| PUR
hot-melt Adhesive – 12 hrs Weatherometer 65°C |
As with other PUR adhesives, PUR hot-melt adhesives rapidly discolor and lose some of its mechanical properties if not protected from UV-induced degradation. A light stabilizer or antioxidant/light stabilizer systems can protect and considerably reduce the amount of discoloration of PUR
hot-melt adhesive.
 Light Stability of PUR HMA Weathering WOM CI 35 (bp 65°C)
Light Stability of PUR HMA Weathering WOM CI 35 (bp 65°C)
Light Stabilizers for Solvent based Adhesives
Light Stabilizers for Solvent based Adhesives
Solvent-based adhesives are susceptible to attack by oxygen and light. Although they are not exposed to very high temperatures during processing, as is the case in the preparation of
hot-melt adhesives, they nevertheless, require stabilization against degradation in end use.
Protection from light is particularly crucial when the adhesive is used in thin films, for instance in tapes, labels or in packaging applications. In these instances, photodegradation due to sunlight, particularly UV light, occurs rapidly. Mainly detected by a yellowing or browning of the adhesive, a loss of adhesive properties can often accompany the color change.
Explore how light stabilizers can improve the resistance of different types of solvent-based adhesives to UV light induced degradation:
Polychloroprene Adhesives
Polychloroprene solvent-based adhesives are found in many market segments because of their excellent adhesive properties. For instance, contact adhesives made of chloroprene films develop rapid bond strength and high level of tack.
Polychloroprene adhesives are generally formulated with an antioxidant to protect the adhesive from oxidation. When used in combination with a UV absorber or HALS, the light stability of chloroprene adhesives can be considerably enhanced.
Light Stability of Chloroprene Solvent-based Adhesives Films (25 µm)
Natural Rubber Adhesives
Natural rubber-based adhesives are generally sold as solutions in a tackifier resin blended with some toluene. Antioxidants are always added to protect the unsaturated backbone from oxidation degradation. With the additional use of a light stabilizer, the adhesive properties and color retention of a solvent-based adhesive based on natural rubber can be further enhanced.
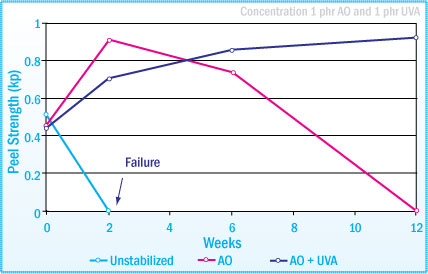 Peel Strength of Natural Rubber-SBA after Daylight Exposure
Peel Strength of Natural Rubber-SBA after Daylight Exposure
Films obtained from the following formulation, 100 parts NR, 80 parts rosin ester and 1000 parts toluene, are exposed to diffuse daylight for a total of 12 weeks. As seen from the images below, with the use of an antioxidant and UV absorber, the adhesive exhibits:
- Enhanced tack retention
- Improved color retention
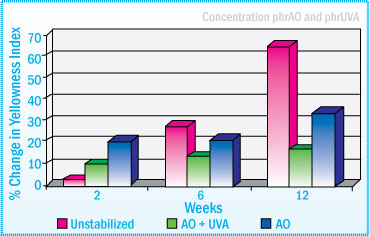 Natural Rubber-SBA: % Change in Color After Daylight Exposure
Natural Rubber-SBA: % Change in Color After Daylight Exposure
Polyurethane Adhesives
Polyurethane adhesives are produced in many grades such as one-component, two-component, dispersion and solvent-based, for use in different application areas. They are suitable for several different industrial and construction applications, having good adhesion to rubber, leather, textiles, metal, paper, wood, and plastics. In addition, solvent-based polyurethane adhesives are used for a wide variety of laminating applications.
Solvent-based polyurethane adhesives consist of a high molecular weight hydroxyl terminated polyurethane dissolved in a solvent. As with other urethane adhesives, aromatic polyisocyanates such as toluene diisocyanate (TDI) and diphenylmethane isocyanate (MDI) are the most used isocyantes and contribute to both the discoloration and deterioration of mechanical properties of the adhesive. The best stabilization results are achieved with the combined use of an antioxidant and UV absorber as seen in the image below.
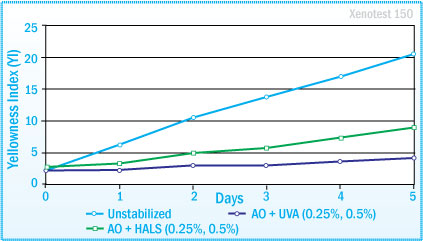 Light stability of Polyurethane Solvent-based Adhesives 100p PUR 30% MEK, 50p MEK, 10p Hardener
Light stability of Polyurethane Solvent-based Adhesives 100p PUR 30% MEK, 50p MEK, 10p Hardener
TPU Adhesives
Thermoplastic polyurethane (TPU) Adhesives are mainly crystalline polymers developed for use as raw materials in bonding applications such as footwear, furniture automotive and others industry.
These products are mainly based or aromatic isocyanates (TDI or MDI) and are highly sensitive to discoloration induced by UV light exposure, so as with other PUR adhesives, TPU adhesives will rapidly discolor and lose some of its mechanical properties if not protected from UV-induced degradation.
A light stabilizer or antioxidant/light stabilizer systems can protect and considerably reduce the amount of discoloration in of TPU adhesive.
» Check Out the Light Stabilizers for Solvent-based Adhesives Here!
Light Stabilizers for Water based Adhesives
Light Stabilizers for Water based Adhesives
Adhesives based on emulsion polymers are gaining in popularity for a number of reasons.
- Switching to water-based adhesives from solvent-based adhesives allows manufacturers to significantly reduce their emissions of volatile organic compounds.
- Emulsion adhesives include the absence of flammable solvents, which not only facilitates mixing and handling, but also reduces the special precautions required for the use of solvent-based adhesives.
Water-based adhesives include resin-based adhesives such as:
- Styrene butadiene rubbers (SBRs)
- Vinyl acetate-ethylene (VAEs)
- Acrylics
Explore how light stabilizers can improve the resistance of different types of water-based adhesives to UV light induced degradation:
SBR Latex
For adhesives and sealants, latex products are being extensively used as alternatives to solvent-based systems. Applications varying from carpet backing, nonwoven, construction and laminating, the range of applications is expanding to meet the needs and requirements of the industry. For pressure sensitive adhesives, SBR latex is being increasingly used in several areas to replace the traditional solvent-based adhesives.
Although latexes normally contain an antioxidant, it is important to ensure that the final adhesive contain sufficient antioxidants to provide adequate protection against thermal oxidative degradation. Furthermore, if the adhesive will receive any exposure to UV light, the addition of a suitable UV absorber and stabilizer is essential. Although SBR latex adhesives generally have good resistance to environmental deterioration, they will not retain good color when exposed to ultraviolet light.
The image below demonstrates how a carboxylated SBR adhesive film discolors after exposure to artificial light after just 3 weeks. With the use of a light stabilizer combination, the adhesive was able to retain much of its color.
 Light Stability of X-SBR Latex Films After Light Exposure
Light Stability of X-SBR Latex Films After Light Exposure
VAE Emulsion
Polyvinyl acetate is most widely used in the form of a dispersion of solid resin in water. More commonly termed emulsions, these dispersions are produced by emulsion polymerization. The largest portion of the adhesive market is served by vinyl acetate and its copolymers. Because polyvinyl acetate is a stiff material, it is often times copolymerized with monomers to provide greater flexibility. The resulting mobility of the copolymers allows for better adhesion to plastic surfaces.
Vinyl acetate-ethylene (VAE) copolymers are an example of this class of emulsion. They have all the benefits of the homopolymer in strength and heat resistance, with the benefit of better adhesion characteristics.
 Discoloration of VAE Emulsion Films After Light Exposure
Discoloration of VAE Emulsion Films After Light Exposure
UV light induced oxidation and subsequent degradation causes loss of adhesive properties such as tack, adhesive or cohesive strength, and discoloration. The following graphs demonstrate the effects of both natural and artificial light and weathering on the color of a VAE emulsion film. In applications requiring critical performance demands, using either a UVA or combination of UVA and a HALS in the adhesive can significantly improve the color retention of the adhesive.
 Discoloration of VAE Emulsion Films After Light Exposure
Discoloration of VAE Emulsion Films After Light Exposure
Vinyl acetate polymer adhesives function best in the bonding of paper and wood and are widely used in paperboard packaging. These adhesives also find use in bookbinding, construction, textiles, envelopes, bags and labels. Furniture uses include wood veneer, edge gluing and general assembly.
Overall, the choice of UV stabilizer for a particular polymer and application is governed largely by economic factors and by technical considerations not related to ultraviolet absorbance. Some of the more important considerations are:
- High solubility of the stabilizer in the polymer
- Low rate of stabilizer loss from the polymer through volatilization, leaching, etc.
- Absence of chemical reactivity of the stabilizer with the polymer or other compounds in the formulation
- Low initial color and good color stability
- Low toxicity
- Ease of compounding
- Lowest possible cost consistent with the desired performance properties
The effectiveness of UV stabilizers can vary from polymer to polymer.
» Discover the Light Stabilizers for Water-based Adhesives Here!
UV Exposure Tests to Determine Material Weatherability
UV Exposure Tests to Determine Material Weatherability
Because the degrading factors of outdoor weathering vary so widely over the earth’s surface, the weathering of materials in not an exact science. It is almost impossible to rank the degenerative power of UV radiation without considering the other elements.
Most laboratory tests are designed to determine the effect of UV radiation alone; however, some tests combine elements. Because of this the estimation of service life by extrapolation methods is significantly risky.
Accelerated testing can, at best, determine the relative effect of various adhesive and sealant formulations to environments that are similar to the service environment being encountered.
Table below common UV exposure tests that are used to determine the weatherability of plastics, coatings, adhesives, and sealants. No laboratory test can completely simulate outdoor weathering conditions (diurnal and seasonal variations, synergistic effects of other degradation mechanisms, specimen design, etc.). Therefore, laboratory test results must be carefully considered relative to specific situations.
| Test |
Characteristics |
| Fluorescent UV / condensation devices (QUV, Atlas UV, Weatherometer) |
Artificial accelerated weathering test to help predict the durability of materials subject to attack by sunlight or moisture. QUV testing simulates the effect of sunlight with fluorescent UV lamps, while it simulates rain and dew with constant humidity. It is much stronger on the high energy, short wavelength region of the spectrum than normal sunlight. In the fluorescent UV apparatus, materials are alternatively exposed to UV alone and condensation alone in a repetitive cycle. |
| Carbon Arc |
Carbon arc devices generally use two lamps with the flame carbon arc open or encased in a borosilicate glass cover that acts as a filter for low wavelength radiation. The spectral distribution exhibits a significant amount of UV below 300 nm. Generally, the device is considered deficient compared to natural sunlight. |
| Xenon Arcs |
Xenon arcs require a combination of filters to reduce unwanted radiation. SAE J1885 uses this test for automotive interiors. |
| FS-40 Lamp (F40-UVB) |
This lamp is specified for coatings (SAE J2020). It has demonstrated good correlation to outdoor exposure for gloss retention and for the integrity of plastics. |
| UVA-340 Lamp |
Simulates UV from 365-295 nm. It has demonstrated good correlation to Florida weathering. |
After artificial aging, the damage done by UV is determined by routine testing.
- In plastics or adhesive joints, the embrittlement produced by ultraviolet radiation can be evaluated by measuring the impact resistance or toughness of the material.
- For tapes, sealants, and pressure sensitive adhesives, the peel strength is generally measured.
Deterioration in appearance can be evaluated by measuring color shift and loss of gloss.
Real-time weathering data from natural environment exposure remains the standard to which all other weathering data is compared. Two of the most commonly used harsh aging sites are Arizona (high ambient temperature and radiation) and Florida (high radiation combines with high humidity).
Find Suitable UV/Light Stabilizers for Adhesives & Sealants
View a wide range of UV/Light stabilizers available in the market today, analyze technical data of each product, get technical assistance or request samples.