Myth #1: Surface Energy & Adhesion
Myth #1: Surface Energy & Adhesion
The first, and biggest myth is that surface energy is important for adhesion. Countless companies spend time trying to get an extra 1 dyne/cm of surface energy in the hope of getting extra adhesion. This extra dyne is a complete waste of time, as we can easily show.
Adhesion can be measured in terms of Work of Adhesion in J/m². This is the work/energy needed to separate 1m² of an adhesive interface. It can also be measured as Peel in N/m, i.e. the force required to pull off a strip 1 m wide. These two measures are identical: 1 J/m² equals 1 N/m.
Go to www.stevenabbott.co.uk/practical-adhesion/basics.php for more details.
Work of Adhesion: Order of Magnitude
To give us some idea of what these units mean, and using round numbers:
This immediately tells us that surface energy is irrelevant. A typical PostIt-style note will have a peel of 4N/m and a strong tape will be 400 N/m. We all know that a typical surface energy of a polymer is 40 dyne/cm which happens to be 40 mN/m. So a Postit-style note is 100x stronger than surface energy and reasonably strong adhesion is 10000x stronger. This makes the hunt for that extra 1 dyne/cm look foolish – which it is.
One area where this hunt is most common is the corona or plasma treatment of films such as polyethylene (PE), polypropylene (PP) or polyester (PET). The faulty logic goes like this: “We get no adhesion onto PE, PP or PET without corona; when we corona treat, the dynes level goes up and we can get good adhesion; therefore the increase of adhesion is due to the dynes.” This is the old trap of saying that correlation equals causation.
So what is really going on? Here’s a clue: the surface energy of amorphous PET is the same as that of PET film. Yet it is very easy to stick to amorphous PET without corona treatment.
The Real Effect of Corona Treatment
To understand what corona (I will use that word as a short-hand to include most common plasma and flame treatments) is doing we need to look at a fundamental bit of adhesion science. Strangely, it starts with the assumption that there is no surface energy adhesion between surfaces, but it rapidly leads to an understanding of how to get significant adhesion.
For reasons that will soon become clear, let us take a sphere of some material of interest with radius R and modulus E* (so-called reduced modulus) and push it with a force F against a flat surface. If there is no attraction between the two surfaces, then we have the Hertzian formula giving the (half) contact width via
a3 = 3FR/4E*
The results of this equation can be seen in the first app: All the apps are free, there are no marketing hooks or registration and they run on everything from phones to laptops and on all operating systems.
The image shows the obvious fact that at zero force there is zero contact width.
But let’s do it in the real world where there is always attraction between surfaces. Now we have to use the famous JKR (Johnson, Kendall, Roberts) formula and include γ the surface energy:
Now you can see why apps are a good idea. You probably don’t have time to work out what that equation means (other than to note that if γ=0 it becomes the Hertz formula), but you would like to see what it’s like in practice. Well, just go to this app and find the following:
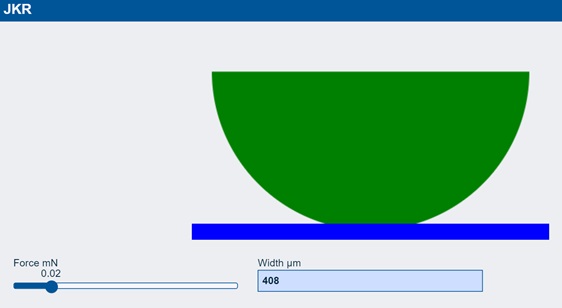
At the same zero force, there is contact width of 408μm. Indeed, as the sphere comes close to the surface it jumps into position as it is positively attracted by surface energy. As you apply more force the width gets wider and when you reverse the force you find that you have to go to -8 mN to pull the sphere away. All this is pure surface energy adhesion which is of no interest to us.
So what does this have to do with real adhesion? If you take a real-world polymer sphere and push it against a surface and measure the contact width, it follows the JKR prediction. But when you try to unload the sphere you find that things have changed. The JKR curve shows “hysteresis” or, what we would call, “adhesion”.
 (Click on Image to Enlarge)
(Click on Image to Enlarge)
The loading curve is the JKR curve with the modest 40 dyne/cm or 0.04 J/m². But on unloading you find a much larger contact width and unlike the pure JKR which separates at -8 mN it takes -148 mN (you can use the mouse to read off the value) to pull the two surfaces apart. This has all happened because in each 1 nm² there is one polymer chain that has crossed the interface and travelled 2 nm into the other polymer. This is a modest “intermingling” of polymer chains and gives us 0.79 J/m² of adhesion. If (not shown) we allow the polymers to “entangle” when they cross the interface then the same 1/nm² and 2 nm gives us 75 J/m², i.e. we have real adhesion. The equations (de Gennes and Lake & Thomas) for these effects are discussed in the apps.
Anyone who has given a sharp tug to a tangle in a ball of string knows the answer: pulling on one bit of string ends up pulling a large amount of string which distorts under the effort. The pulling force becomes dissipated across a wide area and is not focused along the interface itself. It turns out that dissipation is a key to many types of strong adhesion.
The important point in terms of the myths is that if you can get polymer chains to cross an interface and, at first, intermingle then entangle, you get strong adhesion. Conversely, if the polymer surface is so crystalline that the polymer chains cannot move via modest heat or suitable solvent, then you have just surface energy adhesion which is useless.
So that’s the primary function of corona; to reduce the crystallinity at the surface to allow intermingling and entanglement.
The True Effect of Corona Treatment: Proofs
There are two interesting proofs of this.
-
First, if you read the literature on classic heat sealing, where polymers are taken above their melting point, no one disputes that the effect is due to entanglement. But it was observed decades ago that if you press together corona treated PE and warm to 50°C then you get good heat sealing, 70°C below the melting point.
- The second is the realization in the 1980s by 3M, Hoechst and others that treatment of a PET surface with an excimer laser or xenon flash allowed the surface to exhibit high adhesion via solvent coatings or (as with the corona PE) heat sealing at modest temperatures. Experiments showed that there was no degradation or functionalization of the surface – all that had happened was a sudden heating above the melting point then a rapid cooling to give an amorphous surface which is easy to adhere to.
Myth #2: Substrate Wetting
Myth #2: Substrate Wetting
There is another popular argument for wanting a high surface energy via corona. This states that you cannot coat an adhesive unless it wets the surface. This is only a half truth, which gives us a chance to introduce another app:
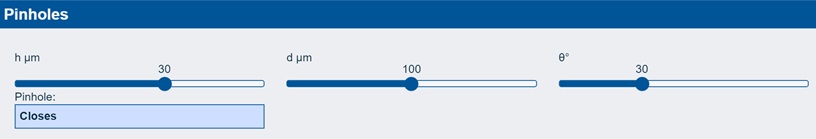
The app explores the idea that if you have a coating of thickness h, a defect of size d and a contact angle of θ, then a hole will open to give you a visible pinhole and bad adhesion if h/d < 2(1-Cosθ).
 (Click on Image to Enlarge)
(Click on Image to Enlarge)
In the example shown, a contact angle of 30° for a 30 µm adhesive coating with a defect of 100µm will not show a pinhole. But (explore this yourself) if the thickness goes down to 25 µm then you have a visible pinhole. So you don’t just care about the dynes level of your corona treatment – you care about the contact angle, the likely size of any defects (e.g. from dust) and whether Marketing are going to ask you to reduce the wet thickness.
Is there any use for measuring dyne levels or contact angles? Yes! If for the past year the surface of a successful product is 40 dyne/cm and today it is 38 that tells you that something is wrong.
Am I admitting that a low dyne level is bad? No, because it is equally the case that if today it is 42 dyne/cm you should be equally worried. “Higher” doesn’t mean “better” – it means that something has changed which in turn means that your process has changed, which generally means trouble.
Now let’s discuss other popular adhesion myths and address the argument about surface functionalization via corona where, again, some half-myths have to be corrected.
Myth #3: Surface Roughness Increases Adhesion
Myth #3: Surface Roughness Increases Adhesion
It is often said that a rougher surface increases adhesion. This is often accompanied by an image from a surface profile meter which might look like the following, taken from my Surface Profile app.
If you imagine an ant walking across the smooth surface (the dashed line at 2.5 µm), then imagine the same ant walking up and down all those mountains and valleys, it seems obvious that it would have to walk much further. And all that extra surface must surely increase adhesion.
 (Click on Image to Enlarge)
(Click on Image to Enlarge)
Yet if you look at all the readouts that the app provides you see that the LR value (Length Ratio) is 1.00, which says that to 2 decimal places, the ant walks the same distance up and down the “mountains”. There is no significant extra surface area to help adhesion!
This make sense if you click the Scale option in the app you get the same data plotted to scale which is 12.5 mm in the X direction and 12500 µm in the Y direction:
Now the flatness of the surface is obvious. In other words, the adhesion community has deceived itself for years by looking at surface roughness profiles without taking into account the scale.
If you examine the scientific literature, the effect on adhesion of roughening most surfaces is very small – which is no surprise. The main exception is the “roughening” of surfaces (e.g. with sandpaper) to remove contaminants. For example, metal surfaces often have layers such as oxides on the surface which can fail mechanically if adhesive is applied to them. Removing those oxides is what increases adhesion, not the extra (minimal) surface area. There is an extreme case of roughening aluminum surfaces electrochemically which gives extreme roughness with 1 µm pillars that are 100 nm wide. These give an increase of adhesion – but only by a factor of 2-3, not a big deal.
Myth #4: The Mechanical Interlocking
Myth #4: The Mechanical Interlocking
There’s another myth that is linked to the roughness myth. It is often said
that rough surfaces give strong adhesion via “mechanical interlocking”. An
authoritative voice from the adhesion world is good enough for me.
Prof Kevin
Kendall, the K in the JKR theory
discussed under surface energy & adhesion, simply states in his marvelous book, Molecular Adhesion and Its Applications: The Sticky Universe, that the mechanical interlocking idea is not only wrong but impossible! It is very frustrating to see as an explanation for the effect of corona/plasma diagrams or AFM plots of “rough” surfaces created by the treatment. These are often stated to provide “mechanical interlocking” with no science to back up the statement and a lack of perspective about how minute the extra “roughness” actually is.
There is an exception. Adhesion to things like paper, board or non-wovens often relies on the adhesive wrapping around the fibers, providing true mechanical interlocking which is very effective. But on “normal” surfaces, the idea is without merit.
There was a trend at one time to believe the (mostly Russian) idea that electrostatics at the interface could provide strength, but again this has proven to be wrong.
» Explore the Basics of Adhesion Theory in Detail!
Myth #5: Chemical Bonds
Myth #5: Chemical Bonds
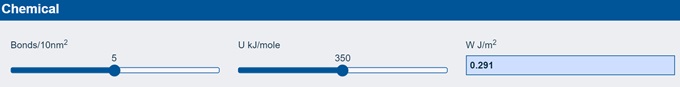
The final half-myth is that chemical bonds provide strong adhesion. It will, therefore, come as a surprise that if you calculate how much adhesion you get from a surface made up entirely of chemical bonds it is less than 1 J/m2. The app does the calculation for you:
 (Click on Image to Enlarge)
(Click on Image to Enlarge)
As you can see, if you have 5 bonds every 10 nm² (and that’s a lot of bonds!) and if the bond strength is a typical C-C or C-O bond ~350 kJ/mole you only get 0.3 J/m². If you don’t believe me, here is a quote from the famous book by Gordon, Structures: or why things don't fall down:
"It turns out that the total energy needed to break all the bonds to any one plane or cross-section in most technologically relevant materials is very much the same and doesn't differ widely from 1 J/m2"
Why are chemical bonds, on their own, so useless? Note the phrase “on their own”. The answer is that Gordon’s 1 J/m² comes from glass. If you put a crack into glass, then it is trivially easy to break all those strong silica bonds across the interface. To take another quote from Gordon: “The worst sin in an engineering material is not lack of strength or lack of stiffness, desirable as these properties are, but lack of toughness, that is to say, lack of resistance to the propagation of cracks”. In the context of adhesion, it means that we often have to focus more on toughness than on raw strength. All those chemical bonds are strong, but they certainly aren’t tough!
Using Chemical Bonds for Strong Adhesion
So how do we use chemical bonds intelligently to get strong adhesion? A good example, which returns to the myths surrounding corona, is the use of PEI (polyethylene imine) as a primer to PE. It happens that PEI is a truly poor polymer in terms of mechanical properties even when (as is the norm) it is lightly pre-crosslinked for these primer applications. And a typical corona treatment might result in just 5% of the surface being functionalized with a mix of -OH, C=O and CO2H groups, with the -OH groups unable to bond with the PEI. And yet for decades products have relied on the PEI reacting with the PE surface then reacting with groups (such as epoxies, urethanes or acrylates) on the other surface to provide strong adhesion. Note that the ideal thickness of the weak PEI layer is <50 nm – thicker coatings are at the risk of cohesive failure within the PEI.
So how does this combination of low levels of functionalization and a weak polymer give strong adhesion? The answer is “entanglement”, just as it is the answer for those systems (discussed above) where polymer chains can cross an interface and become entangled. Here is the remarkably simple, yet profound, definition of entanglement:
If the polymer chain crosses the interface (marked with *) just twice then if you pull on that polymer chain it can slide out. But if it crosses 3 times then attempts to pull it out will be stopped by another chain where it will form a tangle. Whether a polymer chain is stopped by a physical encounter with another polymer chain or a crosslink to that chain makes no difference. So chemical crosslinking gives exactly the same entanglement and both forms absorb the crack energy along the interface by spreading it across the polymer network, dissipating the energy and stopping the crack from propagating.
Why Adhesion Decreases with High Crosslink Density?
And now a point that is often overlooked. In adhesion, too much of a good thing is a bad thing. It has often been noticed that adhesion increases when you start to increase crosslink density, but at a certain point, adhesion decreases with a further increase in crosslinks. This is when you go from a dissipative system to a rigid system which fails, like glass, with 1 J/m². And that is why the low functionalization level of PE and the rather weak PEI are such a great combination – they don’t try too hard!
Another classic example is APTES (3-aminopropyltriethoxysilane). It is a wonderful adhesion promoter between aluminum and epoxies or urethanes. But only at relatively low levels. Increase APTES further and adhesion strength falls off rapidly.
We can summarize what we’ve learned about the myths and point to a more positive view by comparing the “classic” list of adhesion mechanisms to the correct list:
| The Classic List |
The True List |
- Physical adsorption (Surface energy)
- Chemical bonding
- Diffusion – Intermingling/Entanglement
- Electrostatic - wrong
- Mechanical interlocking – wrong, except for paper etc.
|
- Surface energy. Weak and useless except for geckos.
- Chemical bonds. Surprisingly weak and often useless or worse unless entangled.
- Intermingling/Entanglement. This is most of strong adhesion, and chemical crosslinks (but not too many!) are equivalent to entanglement.
- Dissipation. A large element of much of strong adhesion via entanglement and most of PSA.
- Structural. Not discussed here.
|
Going forward, we will build on the theme that dissipation is important for adhesion and see that when it comes to dissipation, time is equivalent to temperature!
No one is going to tell their customer that they have provided a weak adhesive, so they tend to use a different language such as “hot melt” or “laminating adhesive”. In the next section, I will refer to them all as PSA, Pressure Sensitive Adhesives even though the one fact we can all agree upon is that PSA have no significant dependence on pressure!
We will discuss primarily the generic PSA system (such as tapes) with which we are all familiar before extending the science to hot melts or laminating adhesives.
Of course there are many hot melt adhesives which deliver a polymer which is strong in itself and which gets entangled with the adherends. Similarly, some laminating adhesives have special chemical functionalities to provide the necessary entanglement via crosslinks. But a surprising number of strong adhesives (i.e. they don’t break down under their normal intended use) use the dissipative mechanism that is at the heart of all PSA.
Myth #6: Weak Adhesives Cannot Give Great Strength
Myth #6: Weak Adhesives Cannot Give Great Strength
A typical strong polymer has a modulus of 4 GPa. A typical PSA tape must have a modulus <0.3 MPa, so is at least 1000x weaker than a standard polymer. The need for this low modulus which first mentioned by Dahlquist from 3M and is called the Dahlquist criterion. It’s easy to see the reason for the Dahlquist criterion with an app.
We want to stick our adhesive onto a normal surface which has a roughness of height h and a radius R. Surprisingly, most rough surfaces can be approximated with just these two numbers! If the modulus of the PSA is too high, then even with applied pressure you can’t push the adhesive all the way into the rough structure. But if the modulus is less than a critical value, G
c, which also depends on the surface energy W, then the adhesive will spontaneously flow into good contact and give good adhesion:
Gc = W √(R/h3)
In this example the modulus has to be less than 0.2 MPa.
How does a Weak Polymer give Great Strength?
A strong PSA can easily have a peel of 500 N/m, and yet the only thing that has brought the PSA into contact with the surface is surface energy, which is 10000x too weak. The answer is dissipation. To understand this, we need to look at another app which describes the “simple” 90° peel test. The theory was developed by the brilliant theoretician and experimentalist, Kaelble, and has been implemented in the app:
The PSA is being pulled at 90° at the right-hand end of the graph, and that’s where the maximum tensile stress is ~2.2 MPa. If the peel force was fully focused on a weak surface energy interface, it would fall apart instantly. But look what is happening. The tensile stress extends to 0.3mm ahead of the peel and then, amazingly, goes negative – yes, it compresses the PSA. The effects extend out to 1.4 mm. So part of the answer to how PSA work is that the stresses are spread out, diluting the force on any part of the interface. If (try this in the app) you increase the modulus of the adhesive to 2.7 MPa, the forces are concentrated over 0.8 mm – so the harder you try, the worse it can get.
A Closer Look at the PSA Compression Zone
There is more to it than that. It is well-known that you can get good PSA release from some silicone surfaces. People lazily say, “that’s because the surface energy is low”. But this is clearly wrong. You can have some silicone surfaces and some fluoro surfaces with the same low surface energy and still get strong PSA adhesion. It turns out that that the compression zone ahead of the peel is critical to success. On a proper silicone release surface, the whole thickness of the adhesive slides forwards and backwards (this can be seen with fluorescent beads) and there is no dissipation in this zone. But on any other surface (including fluoropolymers) the adhesive in contact with the surface remains fixed and the material above it moves – i.e. there is viscous dissipation.
This is heavy science! But so much of PSA work has been based on intuitions that happen to be wrong. We need to understand what is really going on, and my contribution to this is to use the great science that is out there (and largely ignored) and provide the apps and the explanations so we don’t have to formulate in ignorance.
Role of Peel Rate
There is a big problem with what I’ve just said about a PSA having a maximum peel of, say, 500 N/m. We can guarantee that the same PSA can easily be measured to have a peel of 40 mN/m (i.e. just surface energy). Anyone who has had a plaster removed by a nurse knows this: pulled carefully by yourself and the adhesion is painful, pulled quickly by the nurse and there is no pain. It’s not that the pain is over before it starts, the adhesion is genuinely lower. Similarly, if you take most PSA tapes down to liquid nitrogen temperatures then they peel off easily from the backing tape. This is a handy, little-known trick if you want to examine the pure PSA without its backing sheet.
One of the profound laws of physics is that temperature is equivalent to time. So the nurse’s fast peel is identical to a slow peel done at a lower temperature. In polymer science the Time Temperature Equivalence/Superposition (TTE/TTS) principle is described by the Williams, Landell, Ferry equation, WLF. The equation (not shown here) is a little obscure, but that’s why we have apps. At www.stevenabbott.co.uk/practical-adhesion/wlf.php you will find this example, taken from a famous paper on rubber adhesion (so not directly related to PSA). The data on the left are peel versus peel rate at a range of temperatures. Not surprisingly, they look like unrelated curves. But if you apply a WLF transformation on the right then you see they all follow a single curve.
Why should you care? Because your customers often do strange things to your product. You may have tested it over a “reasonable” temperature range, but if your customer also does a high-speed process such as slitting or sheeting, the adhesion might be the equivalent of that at very low temperatures, i.e. very little.
Here’s the real magic: WLF Factors for your PSA
If you do a temperature/time sweep on your rheometer to measure the G’, G’’ and tanδ (I’m sure you all have great rheometers because formulation without one is very difficult) then the rheometer’s software will give you a WLF to the data. If (and this is very difficult to do in practice) you measured peel at different speeds and temperatures, you find that peel can be fitted to WLF with exactly the same parameters. So a rather straightforward experiment on a modern rheometer (or DMA if you prefer) can tell you what will happen to your peel at whatever temperature/speed your customer chooses to use.
Which gives us an excuse for the final app, on the same WLF page. Suppose you have created a batch of PSA and in your excellent QC lab at 25°C you have determined the peel at a certain speed. Then your customer complains that peel is too low. You politely ask at what temperature they are measuring it and they say 29°C. You simply tell them to measure the peel at a speed 2.2x faster and they will find the same value as yours. I don’t sell rheometers, but if I did I would say that the cost would be recovered by having the WLF factors for your PSA and therefore being able to head off a very expensive customer complaint.
What about hot melts and laminating adhesives? They follow the same rules, but without the need for the 0.3 MPa Dahlquist criterion at 25°C, they need to be 0.3 MPa at their laminating temperature. You can use a higher modulus polymer which, if done carefully, can give you better properties such reduced creep under shear.
And what about entanglement? The weak polymer must show some resistance when it is stretched. A light level of entanglement provides exactly that need. Too much entanglement and you have a “strong” polymer that simply cracks along the interface. Too little entanglement and your PSA is too weak.
How do you formulate for the right level? The starting point, of course, is a rheometer and, if you are very keen, the Luth-Burgers Ideal PSA you can find here.
Finally, I am not saying that you can formulate a great PSA with just a rheometer. But I can say that without a rheometer your chances of formulating a great PSA in an acceptable timescale are very small.
Now to help you get rid of the seventh myth, let me help you by understanding why testing seldom gives us the information we really want about our adhesive system.
Formulators often accept this bad news with a happy smile: at last, they don’t have to keep looking for a test better than the “industry standards” they are required to use.
Myth #7: You can Measure "True" Adhesion at Interface
Myth #7: You can Measure "True" Adhesion at Interface
People fondly imagine that they can measure the “true” adhesion at an interface, but this is a delusion. The reason is that Adhesion is a Property of the System – a phrase that should stand above every formulator’s bench and manager’s desk. An app allows us to see this fact very clearly in a case where we happen to know exactly what the “true” adhesion is, 40 mN/m, pure surface energy.
Battle for Objective Measure of Adhesion System
At one time I was brought into a battle between two brilliant formulators – one a physicist and the other a chemist. They needed an “objective” measure of their novel adhesion system, but the chemist just wanted to use the crude cross-hatch tape test. The physicist objected (rightly!) that this was a barbaric test with little direct relevance to “real” adhesion. He had access to a fancy nanoindenter and wanted to use that for a more objective test.
Two arguments defeated the physicist. The first is that the nanoindenter is imposing a very complex set of forces onto a system (flow, hoop stresses, compression, adhesion failure) and except in special cases it is impossible to extract anything that resembles adhesion:
The second argument was that 100% of the customers only used the tape test because they knew that anything which passed a reasonable test done under reasonable conditions would never fail for their customer.
Various industries have created “standard” tape tests and for all the obvious shortcomings, the test is a robust way of showing, at the very least, if there is a problem with a batch or a formulation.
Now that you have identified the problem, it's time to look at some tests!
Peel Test
Take two pieces of rubber, held together by surface energy, and test them in a peel tester, a lap shear joint and a “butt” joint:
This app allows you to explore what happens. In each case the amount of rubber in contact is similar, but look at the results, which are scientifically correct because they were worked out by (and tested by) Prof Kevin Kendall:
It takes a force of 0.01 N to peel them apart, 2.24 N to pull the lapjoint apart and 50 N to pull the butt joint apart. Which is the “true” force? They all are. Adhesion is a Property of the System and it happens (equations are shown in the app) that the modulus of the rubber plays a large part in the lap and butt joints, giving an instant magnification through purely mechanical means.
Who cares about rubber sticking together with surface energy? Let’s stick a wing onto an aircraft. For this we use a lap shear joint, this time with some adhesive in between the adherends:
It is intuitively obvious that the force to failure will depend linearly on the overlap length L and that the joint will fail in pure shear – that’s why it’s called the lap shear joint. Both “facts” are wrong. It needs a very complex model to show what is going on, but fortunately modern apps are very powerful and the
Goland-Reisner model is a good approximation to the truth:
Points of interest
-
All the forces in the middle are 0 – i.e. all that adhesive in the overlap is doing no good at all!
- There are strong shear forces – not a surprise – but there are even stronger peel forces. In fact, most lap “shear” joints fail via peel rather than shear.
In the early days of modern aircraft, it was worked out that any Al lap joint that failed a lap shear test with a force greater than a given amount was not going to fall out of the sky from adhesive failure. The engineers knew that the test was bogus – it was testing peel whereas the relevant failure mode in the sky was shear. But the test is easy to do and if no planes fall out of the sky, that’s good enough. Until composite aircraft arrived. Give them the same “industry standard” test and they fail catastrophically, even though we know they are actually stronger than aluminum ones in shear. As it happens, composites are very weak in peel, which is why the fail in this “shear” test. The industry had to change to the double-lap shear joint which is much more inconvenient but a better approximation to pure shear.
It is interesting to note that all the inputs to the Goland-Reisner model are mechanical and geometrical – there is nothing about “adhesion”. The model assumes that there is good basic interfacial adhesion and that failure is much more to do with stresses concentrated at the end of the joint. Adhesion is a Property of the System.
Challenges with Peel Test
The peel test is full of problems. If, for example, the backing tape is rather too weak then a lot of the “peel” is just stretching of the backing. And as discussed previously (look up the Kaelble app if you missed it) there are many things going on at the same time, especially if you do a 180° peel which involves lots of shear forces.
PSA scientists tend to love the “probe tack test” as it has lots of inputs and outputs to study.
The image gives you some idea of the complexity of what happens when you pull the probe away from the sample. The trouble is that this is a “butt” test and as we saw, but tests have no relation to other tests. The failure is “all at once” whereas a typical PSA fails along a peel line.
It turns out that the PSA lab needs rheology, peel, probe tack and, probably, tensile testing to be able to understand why a formulation is bad or good, and how to make it better. Knowledge is power: if you can characterize your PSA via a broad set of scientific measures, you will have the power to develop superior formulations.
Finally, if we look at the thin film industry, there are all sorts of fancy tests that claim to measure “adhesion”. A good example is the 4-point bend test, of which there are at least two versions, explained in the app:
The problem is that to calculate the fracture energy, G, you need a horrible formula: G=[F2L2/(E2b2h3)](6η3-1/2I) and the I in the formula is given by I=Σ[(Δ-1/η)2-(Δ-1/η)+1/3]+Δ/η(Δ-1/η)+1/3η3. It doesn’t matter what all this means (it’s explained in the app), the important thing is that an “objective” measure of adhesion requires a complex set of numbers which makes it not at all obvious what is going on.
Rolling Ball Test
One area that is desperate for an objective measure of adhesion is Pressure Sensitive Adhesives, PSA. But, as discussed previously, a PSA is a hugely complex system so there is no single test which shows that it is fit for purpose. Rheology provides a basic grounding – if it is not in the right part of rheology space it will not work. But some formulators try to formulate with little more than a rolling ball test:
It is important to note that this test has no scientific basis because the ball is moving along the tape at highly variable speeds, so the temperature/time issues are totally mixed up. It is a great QC test to say that this batch is the same as the last one, but if the distance changes there is no way to know which aspect of the formulation has gone wrong.
Similarly, the loop tack test is so complex that there is little scientific merit in using it for formulation, though it is a handy QC test.
Sorry to depress you with the negatives associated with these tests. Once you grasp that “Adhesion is a Property of the System” and that no single test is ever good enough, the result is quite liberating. You use whatever test gives you the most formulation insight (and industry-accepted data) for the least effort, always keeping in mind that properties such a modulus might be much more important than “adhesion”. Good luck with your testing!
My mission here is to use some of the previous themes to explore how we can ensure low adhesion (abhesion) for the times that we require it, and at the same time find ways to identify why unwanted adhesion failure occurred.
Because we know that surface energy is of no importance to real adhesion (other than its modest role in ensuring a good coating of a liquid adhesive) we don’t have to waste any time discussing it.
Instead for those who want abhesion, here is a set of proven methods, taken from my webpage:
- Provide a “sharp” interface
- It is relatively easy to have a "sharp" interface by either having crystalline materials (un-corona-treated PE) or by ensuring no intermingling or entanglement via incompatibility (for polymers use: Hansen Solubility Parameters to maximize the "Distance" between them) and/or from making sure there's insufficient temperature/time for intermingling to take place. With a sharp interface, all the energy of a crack is focused on a sub-nm scale and failure is guaranteed.
- Provide no chemical entanglements
- This is similar to the previous one - just have too few chemical bonds and/or make sure that they are not coupled into some sort of entangled network.
- Provide too much chemical entanglement
- Conversely, make sure there is so much cross-linking that the system is brittle, ensuring little dissipation and therefore "only" chemical bonds which are in the range of 1J/m2.
- Prepare the surface badly
- Surface junk through under-cleaning or from over-zealous corona/plasma will give poor adhesion. Roughening is nearly always useless at best (the extra surface area provides negligible extra adhesion) and can often be positively harmful if the adhesive cannot flow into the structure.
- Arrange the mechanics badly
- If you are involved in structural adhesion, then focusing all the forces onto the weakest spot will ensure bad adhesion.
- Use the wrong temperature/time
- An adhesive which is perfect at 40° or tested at 1Hz might be perfectly useless at 0° or 100Hz. Time Temperature Superposition/William-Landell-Ferry should help you get the correct (bad!) combination of factors for abhesion.
That little list is also helpful for when failure is not an option. The most important lesson to draw from it is “Don’t try too hard”. Let me give three areas of adhesion where trying to hard made matters worse. You can readily apply the lessons to your own area.
Myth #8: More UV Curing Means Stronger Adhesion
Myth #8: More UV Curing Means Stronger Adhesion
I once found myself in the middle of an adhesion crisis of a product I didn’t know on a machine I didn’t know run by a (great) team I didn’t know. The key component was UV cured and adhesion was failing. All the usual suspects were tried. I then suggested reducing the UV power. This was heresy – more UV means more cure means stronger adhesion. In the end, they had nothing to lose and reduced the power. Problem solved. On careful analysis it was clear that their tough coating was being converted to a strong but brittle coating by excess UV and that’s what caused the failure.
Myth #9: Too much Chemical Primer is Good for Adhesion System
Myth #9: Too much Chemical Primer is Good for Adhesion System
It is always tempting to add a little more chemical primer/crosslinker “just in case”. Stronger is always better, isn’t it? But no. More adhesive systems have been damaged by too much primer/crosslinker than by too little. There are plenty of academic papers showing this and there are all too many industrial users (including myself!) who have found out the hard way. It is the same story – trying too hard makes the system brittle.
Myth #10: Stronger Epoxies Work Better
Myth #10: Stronger Epoxies Work Better
The world of structural adhesives really needs high modulus adhesives. Early epoxies were of modest modulus, but ingenious formulators gradually made them stronger, to great effect. But they hit a limit. Even stronger epoxies made matters worse – the joints became susceptible to brittle failure. The solution was to add some rubber spheres (with a hard shell) to the epoxies. These somewhat reduced the overall modulus yet acted as shock absorbers for cracks.
Problems with Adhesion
- Our biggest problem with adhesion is predicting the effects of time and temperature. To a modest extent these are predictable and can be used to our advantage.
- If we know a customer will subject our adhesive system to a high-speed process we cannot simulate in the lab, we simply do our standard test at low temperatures, using Time Temperature Superposition to tell us what temperature will mimic the customer’s conditions. Similarly, if the customer is concerned about long-term shear issues we can (with due recognition that a lot of “shear” is actually “peel”) use a higher temperature to simulate the long timescale. In both cases, the effects are likely to be “WLF” rather than “Arrhenius”. WLF and its app has been discussed earlier. Arrhenius is the old rule of thumb that increasing temperature by 10°C doubles the rate at which things happen. A 50°C increase in temperature will cause a 32x increase in rate via Arrhenius but for WLF, depending on where you are in the curve, it could be a factor of 1000 or a factor of 2!
- The problem we all face is not so much that of temperature but that of humidity. Water is a small, reactive molecule that can readily diffuse to the interface and destroy a key primer bond (“interfacial failure”) or simply swell the whole system and through cycles of stresses induced by swelling, damage the joint mechanically. Because water effects (unlike thermal effects) are so specific, there is no convenient app to unleash.
However, when I teach adhesion science courses a lot of interest is always aroused when I mention a technique I once came across called ASAP, Accelerated Stability Assessment Program, originally developed by Dr Kenneth Waterman and colleagues at Pfizer for modelling ageing characteristics of pharmaceuticals. Yes, the pharma industry has as difficult a time as we do in predicting long-term effects of temperature and humidity. I have no personal examples of using ASAP in the world of adhesion, but Dr Waterman, now at his own company, provides many free on-line explanations of the technique which seems amazingly powerful, though still targeted largely at the pharma world. The key is that it generates meaningful long-term failure data in (ideally) less than 14 days, bypassing those 60-day “accelerated ageing” tests that yield so little information for so much time spent. It can be implemented at no cost via a simple Excel sheet (contact me if you would like an example that I created for my adhesion courses) or via much more sophisticated statistical techniques to extract the most predictive data for the least experimental effort.
Lessons to Remember
Adhesion is a Property of the System
The next lesson is to remember that “Adhesion is a Property of the System” so think of the whole system, not just your adhesive layer. A key example is the lap shear joint where a systems analysis shows that failure is via peel. And an adhesive which is perfect for one temperature/speed combination will shift to totally unsatisfactory adhesive at a different combination.
Failure Comes in Many Modes
At the same time, broaden you mind from the old “failure is adhesive or cohesive” binary system. Failure comes in many modes, here are 6 of them:
Adhesive Failure and Interfacial Failure
It is very hard to distinguish between adhesive failure and interfacial failure, but it is important to do so. Interfacial failure (often through humidity) is of a bond of a primer molecule to a rigid surface such as glass or metal. Substrate failure is relatively rare and is a strange outcome of the laws of fracture mechanics. I was shocked when I first saw it in a coating on PET film! Near interface failure is when the adhesive and adherend are influencing each other in an undesirable way. And, of course, dissipative failure is often a good thing as it provides the strength of PSA and a lack of it provokes brittle failure.
Finally, I want to summarize some key themes:
- There are a lot of unnecessary myths in adhesion science that should be unlearned as soon as possible.
- Adhesion is a Property of the System, so think of the system not just the adhesive.
- Time and temperature are equivalent, so be alert to that duality.
- Dissipation is often more important than mere strength.
- Too much of a good thing is a bad thing.
- Tests of “adhesion” are often not measuring what you think they measure.
- And, most importantly, with a large set of free, powerful apps to help you master the science, you can develop better formulations faster.
Learn more by visiting Prof Abbott’s “Practical Science” series here or from his book, Adhesion Science: Principles and Practice, DesTech 2015.
 Follow Exclusive Science-based Formulation Channel
Follow Exclusive Science-based Formulation Channel
 Join Science-Based Formulation Community (SBFC) on LinkedIn
Join Science-Based Formulation Community (SBFC) on LinkedIn
About Steven Abbott
 Prof. Steven Abbott, Director of Steven Abbott TCNF Ltd. focussing on Technical software, Coating/Printing, Nano-expertise and Formulation, has been named as fellow of the Royal Society of Chemistry. His research and product development focus on polymer-based thin-layers, coatings and adhesion.
Prof. Steven Abbott, Director of Steven Abbott TCNF Ltd. focussing on Technical software, Coating/Printing, Nano-expertise and Formulation, has been named as fellow of the Royal Society of Chemistry. His research and product development focus on polymer-based thin-layers, coatings and adhesion.
He is passionate about getting products right through development and production to real sales and using technical software to transform theory into hands-on tools for exploring "what ifs" when the various complex parameters change.
AbbottApps now cover PracticalAdhesion, PracticalSurfactants, PracticalFoam, PracticalSolubility, lots of web handling and, well, lots of other things. All Apps are free and run on all platforms. He uses his complex mixture of past skills to help solve new problems.
Specialties: Chemistry formulations, technical software development, nanostructures by the kilometre, Hansen Solubility Parameters, Organic Photovoltaics, Skin permeation